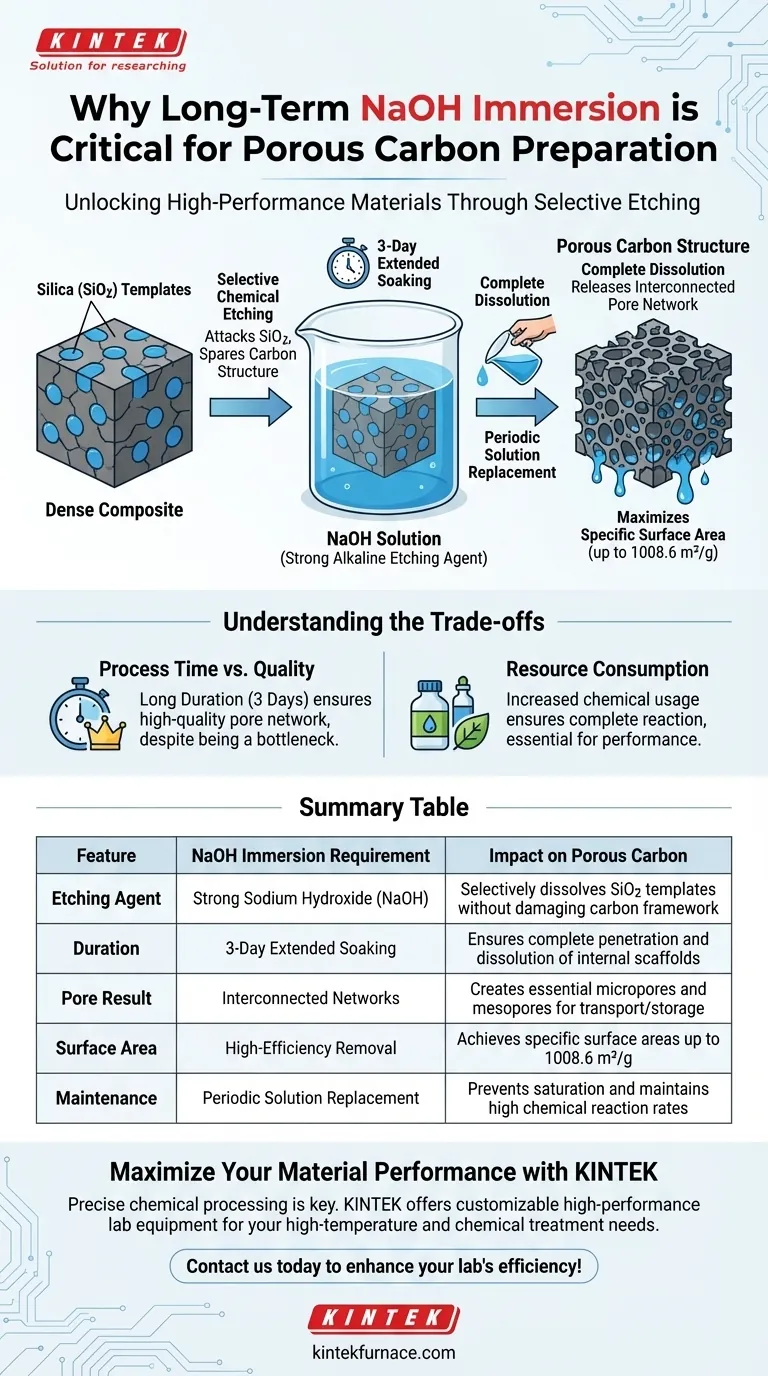
Long-term immersion in sodium hydroxide (NaOH) is a critical processing step designed to selectively remove hard templates from a carbon matrix. Specifically, the NaOH acts as a strong alkaline etching agent that targets and dissolves silica (SiO2) structures embedded within the material. This extended treatment is necessary to ensure the silica is completely eliminated, which reveals the final porous structure of the carbon.
The immersion process is not merely a wash; it is a chemical transformation that converts a solid composite into a highly porous material by dissolving internal silica templates to unlock interconnected voids and maximize surface area.

The Mechanism of Pore Creation
Selective Chemical Etching
The primary role of NaOH in this context is to act as a strong alkaline etching agent. It chemically attacks the silica (SiO2) without degrading the surrounding carbon structure.
This selectivity is vital. It allows for the precise removal of the temporary scaffold (the hard template) while preserving the integrity of the carbon framework.
Unlocking the Pore Network
As the silica templates are dissolved, they leave behind voids where the solid material once stood.
This process "releases" a network of interconnected micropores and mesopores. These connected pathways are essential for the material's performance in transport or storage applications.
Maximizing Specific Surface Area
The removal of the template is directly responsible for the material's high surface area.
According to data on Nitrogen-doped Porous Carbon (RMF), this process is essential for achieving a specific surface area as high as 1008.6 m²/g. Without the complete removal of silica, these internal surfaces would remain inaccessible.
The Necessity of Duration and Maintenance
Ensuring Complete Dissolution
The process requires soaking the material for three days.
This extended duration is not arbitrary; it provides sufficient time for the alkaline solution to permeate the matrix and react with every part of the silica template. Shortening this timeframe risks leaving residual silica, which would block pores and reduce surface area.
Maintaining Chemical Potency
The protocol involves periodically replacing the NaOH solution during the three-day soak.
As the silica dissolves, the solution can become saturated, reducing the reaction rate. Refreshing the solution ensures the etching agent remains at a high enough concentration to drive the dissolution process to completion.
Understanding the Trade-offs
Process Time vs. Quality
The most significant trade-off in this method is time efficiency.
A three-day immersion step represents a substantial bottleneck in manufacturing throughput. However, skipping or shortening this step directly compromises the quality of the pore network.
Resource Consumption
The requirement to periodically replace the solution increases chemical consumption.
This ensures maximum performance but adds to the material cost and waste management requirements of the production process compared to single-wash methods.
Making the Right Choice for Your Goal
When optimizing the preparation of porous carbon structures, consider the following:
- If your primary focus is maximizing surface area: You must adhere strictly to the long-term, multi-day etching protocol to ensure 100% removal of the silica template.
- If your primary focus is process speed: You will need to investigate alternative etching agents or higher concentrations, but be aware that reducing time often results in residual template material and lower pore connectivity.
Ultimately, the long-term NaOH treatment is the defining step that transforms a dense composite into a high-performance, high-surface-area functional material.
Summary Table:
| Feature | NaOH Immersion Requirement | Impact on Porous Carbon |
|---|---|---|
| Etching Agent | Strong Sodium Hydroxide (NaOH) | Selectively dissolves SiO2 templates without damaging carbon framework |
| Duration | 3-Day Extended Soaking | Ensures complete penetration and dissolution of internal scaffolds |
| Pore Result | Interconnected Networks | Creates essential micropores and mesopores for transport/storage |
| Surface Area | High-Efficiency Removal | Achieves specific surface areas up to 1008.6 m²/g |
| Maintenance | Periodic Solution Replacement | Prevents saturation and maintains high chemical reaction rates |
Maximize Your Material Performance with KINTEK
Precise chemical processing is the key to unlocking the full potential of your porous carbon structures. Backed by expert R&D and manufacturing, KINTEK offers high-performance lab equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique high-temperature and chemical treatment needs.
Whether you are refining your silica etching protocol or scaling up carbon synthesis, our technical experts are here to provide the precision tools you require. Contact us today to enhance your lab's efficiency!
Visual Guide
References
- Qi Chen, Licheng Ling. Enhanced Electrochemical Performance of Dual-Ion Batteries with T-Nb2O5/Nitrogen-Doped Three-Dimensional Porous Carbon Composites. DOI: 10.3390/molecules30020227
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- What is the primary function of a vacuum drying oven? Key to Composite Anode Slurry Preparation
- What is the mechanism of solution treatment on Cu-Cr-Zr-La alloys? Master the Thermal Cycle for High-Strength Alloys
- Why is a laboratory constant temperature drying oven necessary for biomass adsorbents? Ensure Precision & Integrity
- What is the specific purpose of using a laboratory oven for the treatment of copper oxide precipitates? Expert Insights
- Why is a high-precision furnace required for Li22Sn5 synthesis? Ensure Pure-Phase Alloy Stability
- What is the function of a drying oven in the chemical activation of biochar with phosphoric acid? Optimize Biochar Quality
- What type of furnace was chosen for annealing silicon-based materials and what were the key requirements? Discover the Ideal Solution for Precise Heat Treatment
- Why is SF6 gas utilized as the primary inhibitor in AS-ALD on ZrO2? Master Defect-Based Passivation Strategy



















