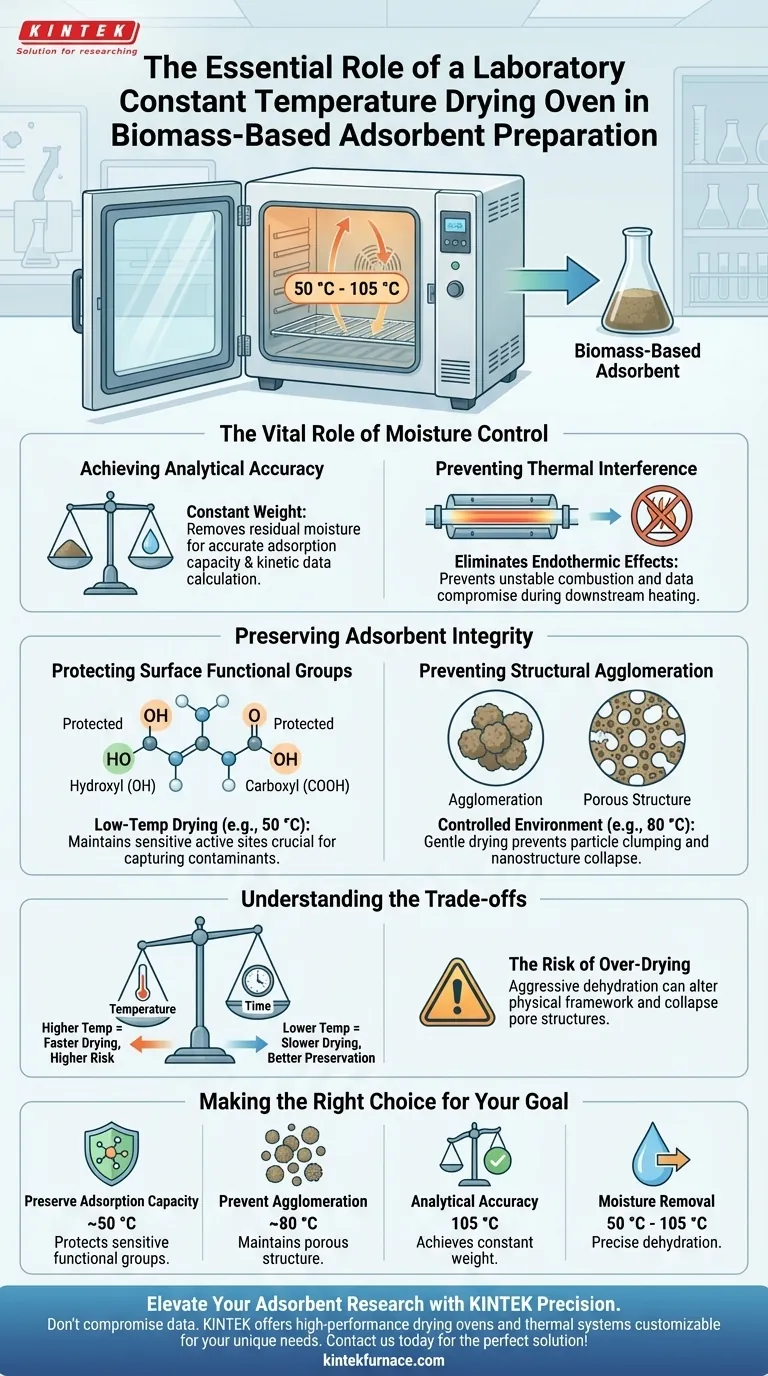
The laboratory constant temperature drying oven is a prerequisite for preparing biomass-based adsorbents because it ensures the precise removal of moisture without compromising the material's chemical structure. By providing a stable thermal environment, usually between 50 °C and 105 °C, it allows the material to reach a "constant weight" for accurate analysis while preventing thermal degradation of the active sites responsible for adsorption.
The core value of this equipment lies in its precision: it balances the aggressive need to dehydrate samples with the delicate requirement to preserve heat-sensitive surface functional groups like hydroxyl and carboxyls.

The Vital Role of Moisture Control
Achieving Analytical Accuracy
In scientific preparation, "dry" is a quantitative standard, not just a physical state. The drying oven is critical for bringing biomass materials to a constant weight.
Without this stability, residual moisture creates variable baselines in weight measurements. This makes it impossible to accurately calculate adsorption capacity or kinetic data later in the experiment.
Preventing Thermal Interference
Moisture acts as a heat sink. If water remains in the sample during high-temperature downstream processes (like tube furnace heating), it causes endothermic effects.
These effects destabilize the combustion temperature and compromise data reproducibility. A pre-treatment at 105 °C ensures physically adsorbed moisture is removed, eliminating this variable.
Preserving Adsorbent Integrity
Protecting Surface Functional Groups
Biomass adsorbents rely on specific surface chemistries, primarily hydroxyl and carboxyl groups, to capture contaminants.
These groups are thermally sensitive. The constant temperature oven allows for lower-temperature drying (e.g., 50 °C) that removes water but maintains the integrity of these active sites. Unregulated heating could denature or destroy these groups, rendering the adsorbent ineffective.
Preventing Structural Agglomeration
The physical structure of the adsorbent is just as important as its chemistry. Rapid or uneven heating can cause particles to clump together.
A controlled environment (e.g., 80 °C) ensures a gentle drying process. This keeps the material loose and prevents the agglomeration of nanostructures, ensuring the precursor remains porous and ready for calcination.
Understanding the Trade-offs
Temperature vs. Time
There is an inherent trade-off between the speed of drying and the quality of the final material.
Raising the temperature accelerates moisture removal but exponentially increases the risk of damaging the biomass structure. Lower temperatures preserve integrity but require significantly longer duration (often 12 to 18 hours) to achieve constant weight.
The Risk of Over-Drying
While moisture removal is the goal, aggressive drying can alter the physical framework of the biomass.
Extreme dehydration can sometimes lead to the collapse of pore structures. It is vital to adhere to the specific temperature protocols (e.g., 50 °C for washing stabilization vs. 105 °C for fuel sample prep) to avoid altering the material's fundamental properties.
Making the Right Choice for Your Goal
To maximize the effectiveness of your biomass-based adsorbent, tailor your drying protocol to the specific stage of preparation:
- If your primary focus is preserving adsorption capacity: Use a lower setting (approx. 50 °C) to dry raw materials and stabilized products, prioritizing the protection of hydroxyl and carboxyl groups.
- If your primary focus is preventing agglomeration: Maintain a moderate, constant temperature (approx. 80 °C) to ensure the precursor remains loose and prevents nanostructure clumping.
- If your primary focus is data reproducibility for thermal analysis: Use a higher setting (105 °C) for at least 12 hours to eliminate all physically adsorbed moisture and prevent endothermic interference.
Success in adsorbent preparation ultimately depends on using the oven not just as a heater, but as a precision tool for chemical preservation.
Summary Table:
| Drying Goal | Recommended Temp | Primary Benefit |
|---|---|---|
| Preserve Adsorption Capacity | ~50 °C | Protects sensitive hydroxyl and carboxyl functional groups |
| Prevent Agglomeration | ~80 °C | Maintains porous structure and prevents nanostructure clumping |
| Analytical Accuracy | 105 °C | Achieves constant weight and removes endothermic interference |
| Moisture Removal | 50 °C - 105 °C | Precise dehydration without compromising chemical structure |
Elevate Your Adsorbent Research with KINTEK Precision
Don't let inconsistent thermal processing compromise your research data. Backed by expert R&D and manufacturing, KINTEK offers high-performance drying ovens, Muffle, Tube, Rotary, Vacuum, and CVD systems designed to protect your sensitive biomass materials. Whether you need to preserve functional groups or prevent structural agglomeration, our laboratory solutions are fully customizable for your unique needs.
Ready to optimize your material preparation? Contact us today to find the perfect thermal solution!
Visual Guide
References
- Yiping Guo, Guoting Li. Coadsorption of Tetracycline and Copper(II) by KOH-Modified biomass and biochar Derived from Corn Straw in aqueous Solution. DOI: 10.3390/w17020284
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- How does the speed-controlled motor in a high-pressure autoclave influence the yield of glucose from starch?
- What is Skin Depth and how does it affect induction heating? Master Frequency Control for Precise Heat
- Why are ceramic materials preferred for dental restorations? Discover Their Aesthetic, Strength, and Biocompatibility Benefits
- What functions does ammonia (NH3) perform beyond acting as a nitrogen source? Unlock Advanced Surface Engineering
- What are the advantages of using a batch furnace? Achieve Unmatched Process Flexibility and Precision
- What role does sodium silicate (Na2SiO3) play as a phase transition additive? Optimize Molten Salt Separation
- What are the primary process objectives of using an infrared belt furnace? Optimize TOPCon Solar Cell Metallization
- Why is cordierite selected as the honeycomb support for HAN decomposition catalysts? Essential Design Insights



















