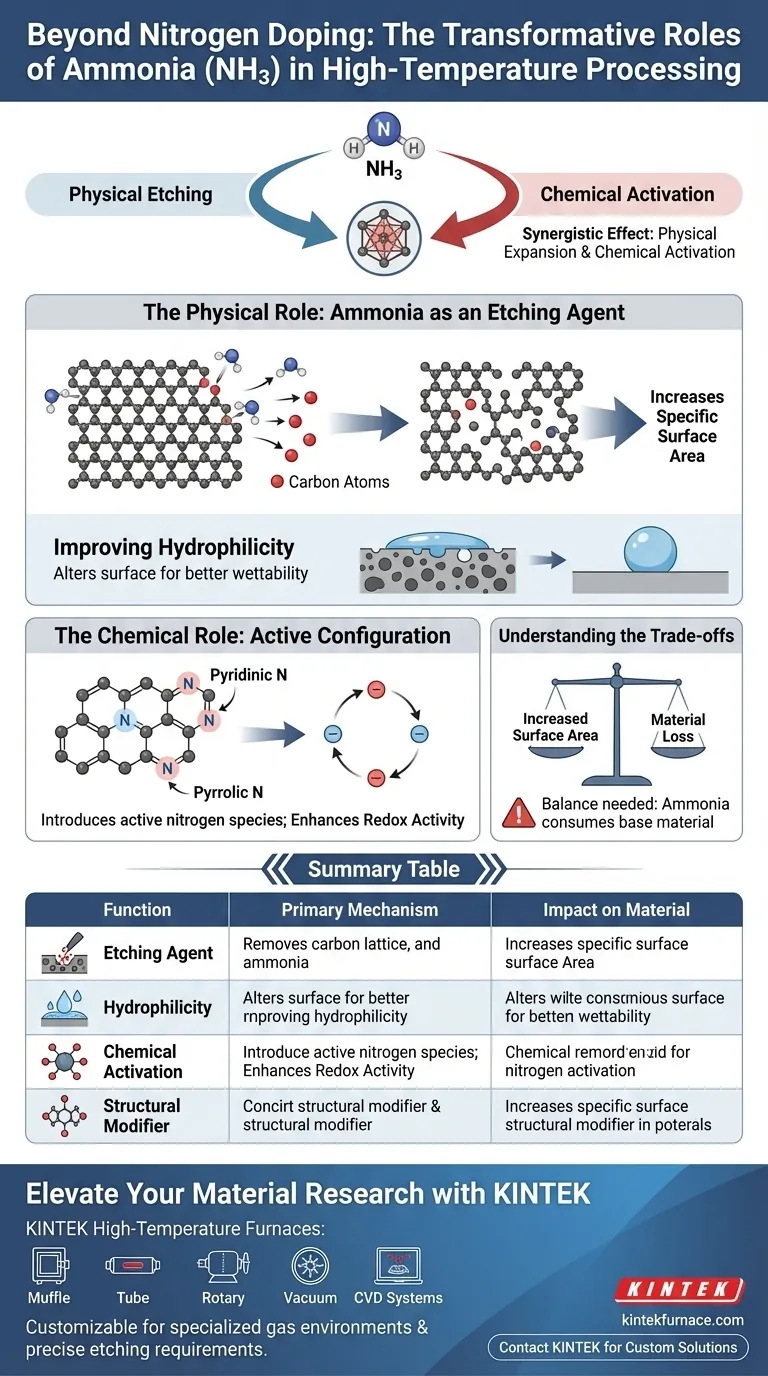
Beyond its role as a dopant, ammonia (NH3) functions primarily as a significant etching agent during high-temperature processing. While it introduces nitrogen into the material lattice, it simultaneously triggers a physical restructuring of the material, actively consuming carbon to create porosity and altering surface chemistry to enhance wettability.
The true power of ammonia lies in its synergistic effect: it physically expands the material's active surface area through etching while chemically activating that surface with specific nitrogen configurations.

The Physical Role: Ammonia as an Etching Agent
Increasing Specific Surface Area
In high-temperature environments, ammonia does not simply sit on the material; it reacts aggressively with it.
Acting as an etching agent, NH3 removes carbon atoms from the material structure.
This process creates voids and defects, significantly increasing the specific surface area of the material.
Improving Hydrophilicity
The structural changes caused by ammonia processing directly affect how the material interacts with liquids.
The combination of increased surface roughness (from etching) and chemical alteration makes the resulting carbon material more hydrophilic.
This improves the material's wettability, allowing for better interaction with electrolytes or other liquid mediums.
The Chemical Role: Active Configuration
Introducing Active Nitrogen Species
While you know NH3 acts as a nitrogen source, the type of nitrogen it introduces is critical.
Ammonia processing specifically favors the formation of pyridinic and pyrrolic nitrogen configurations.
These are considered "active" configurations, distinct from generic nitrogen doping, and are highly sought after for catalytic applications.
Enhancing Redox Activity
The presence of these specific nitrogen groups creates a higher density of functional sites on the material's surface.
These sites facilitate electron transfer, directly improving the material's redox activity.
This makes the material significantly more effective in applications requiring rapid reduction-oxidation reactions.
Understanding the Trade-offs
Managing Material Loss
Because ammonia acts as an etching agent, it inherently involves the consumption of the base material.
Extended exposure or excessively high temperatures can lead to significant mass loss.
Operators must balance the need for increased surface area against the structural integrity and yield of the final product.
Making the Right Choice for Your Goal
To utilize ammonia effectively, you must align the processing parameters with your specific material requirements.
- If your primary focus is maximizing active sites: Prioritize NH3 processing to specifically target the formation of pyridinic and pyrrolic nitrogen groups, which drive redox activity.
- If your primary focus is increasing porosity: Leverage the etching properties of NH3 to strip away carbon and expand the specific surface area for better physical interaction.
Ammonia is not just an additive; it is a transformative tool that reshapes both the physical architecture and chemical potential of your material.
Summary Table:
| Function | Primary Mechanism | Impact on Material |
|---|---|---|
| Etching Agent | Reacts with and removes carbon atoms | Increases specific surface area and creates porosity |
| Hydrophilicity | Alters surface roughness and chemistry | Improves wettability and interaction with liquids |
| Chemical Activation | Favors pyridinic/pyrrolic N configurations | Enhances redox activity and electron transfer |
| Structural Modifier | Creates voids and physical defects | Physically expands active surface area |
Elevate Your Material Research with KINTEK
Precision matters when managing the aggressive etching and chemical transformation of ammonia processing. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems designed to handle specialized gas environments with unmatched stability.
Backed by expert R&D and world-class manufacturing, our lab high-temperature furnaces are fully customizable to meet your unique etching and nitrogen-doping requirements. Don't settle for generic results—optimize your porosity and redox activity today.
Contact KINTEK to find your custom furnace solution
Visual Guide
References
- Xing Huang, Dessie Ashagrie Tafere. Waste-derived green N-doped materials: mechanistic insights, synthesis, and comprehensive evaluation. DOI: 10.1039/d5su00555h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- Why are high frequencies used in induction heating? For Precise, Rapid Surface Heating
- Why is an environmental laboratory chamber equipped with an optical window required for synthesizing Hafnium Carbide?
- What is the role of a customized drying station with nitrogen purging? Optimize Polymer Blend Membrane Processing
- What is the purpose of a high-temperature calcination furnace in Sol-Gel? Achieve High Purity and Crystallinity
- What functions does glucose perform in lithium-ion sieve synthesis? Enhance Carbothermal Reduction for LiMnO2 Purity
- What is the purpose of performing a 600 degree Celsius annealing treatment? Enhance AZO Thin Film Stability
- What are the technical functions of carrier gases in VTD? Master Vapor Transport Deposition Control
- What is the purpose of using a laboratory blast drying oven at 107°C for 17 hours for reforming catalysts?



















