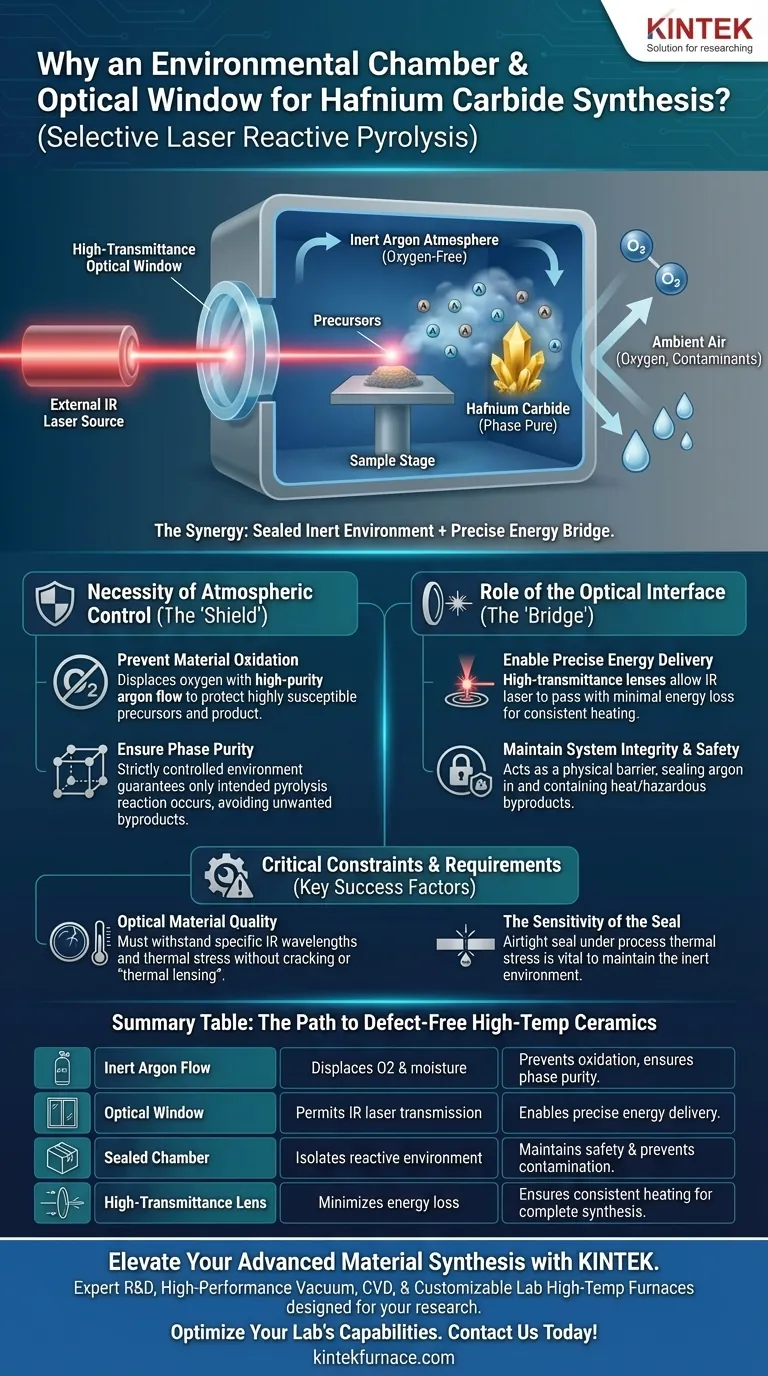
Precise atmospheric control is the critical variable in synthesizing Hafnium Carbide via Selective Laser Reactive Pyrolysis. The environmental chamber isolates the reaction in a high-purity argon atmosphere to prevent oxidation, while the optical window serves as a transparent yet sealed bridge, allowing the infrared laser to deliver energy to the precursors without compromising the inert environment.
The synthesis of Hafnium Carbide requires extreme heat in an oxygen-free environment to achieve phase purity. The chamber seals out contaminants, while the optical window bridges the gap between the external energy source and the internal reactive process.
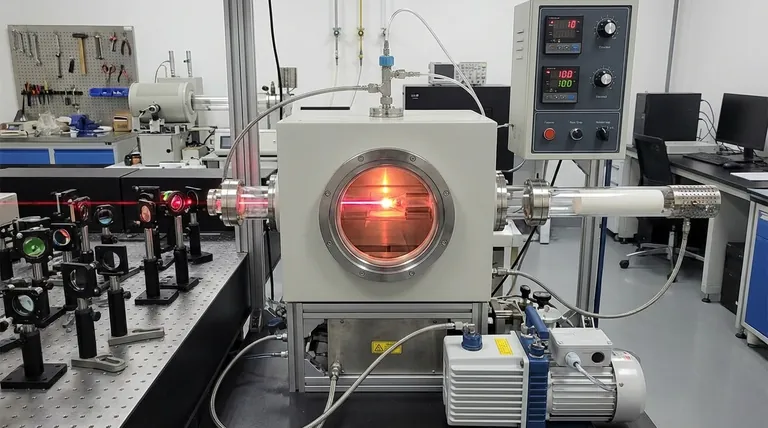
The Necessity of Atmospheric Control
Preventing Material Oxidation
The primary function of the environmental chamber is to create and maintain a controlled inert atmosphere.
During high-temperature laser processing, both the starting precursors and the synthesized Hafnium Carbide are highly susceptible to oxidation.
By flooding the chamber with high-purity argon flow, the system displaces oxygen that would otherwise degrade the materials.
Ensuring Phase Purity
The quality of the final ceramic product depends entirely on the chemical environment during synthesis.
If the atmosphere is not strictly controlled, unwanted chemical reactions will occur.
The chamber ensures high phase purity by guaranteeing that the only reaction taking place is the intended pyrolysis, not combustion or oxidation.
The Role of the Optical Interface
Enabling Precise Energy Delivery
The optical window is not merely a viewport; it is an active component of the energy delivery system.
Typically equipped with high-transmittance lenses, this window allows the infrared laser to pass through the chamber wall with minimal energy loss.
This ensures the laser can precisely reach and heat the sample to the necessary reaction temperatures.
Maintaining System Integrity and Safety
The window allows for laser interaction while keeping the physical barrier of the chamber intact.
It maintains the safety of the operation by containing any potentially hazardous byproducts or heat within the vessel.
Simultaneously, it prevents the high-purity argon from escaping and ambient air from entering.
Critical Constraints and Requirements
Optical Material Quality
The success of this process relies heavily on the specific properties of the optical window.
It must be made of materials capable of handling the specific wavelength of the infrared laser without absorbing excessive heat or cracking.
Low-quality lenses can lead to thermal lensing or energy attenuation, resulting in incomplete synthesis.
The Sensitivity of the Seal
The interface between the optical window and the chamber is a potential point of failure.
This seal must remain airtight even under the thermal stress of the process to maintain the inert argon environment.
Any breach at this junction compromises the oxidation protection, rendering the synthesis failed.
Ensuring Success in Ceramic Synthesis
To maximize the quality of your Hafnium Carbide synthesis, you must prioritize the integrity of your chamber setup.
- If your primary focus is Phase Purity: Ensure your argon flow is continuous and high-purity to eliminate any trace of oxygen during the heating cycle.
- If your primary focus is Energy Efficiency: Verify that your optical window utilizes high-transmittance lenses matched specifically to your laser's infrared wavelength.
The synergy between a sealed inert environment and a high-quality optical path is the only way to achieve defect-free high-temperature ceramics.
Summary Table:
| Feature | Function in Synthesis | Benefit to Final Material |
|---|---|---|
| Inert Argon Flow | Displaces oxygen and moisture | Prevents oxidation and ensures high phase purity |
| Optical Window | Permits IR laser energy transmission | Enables precise energy delivery to precursors |
| Sealed Chamber | Isolates the reactive environment | Maintains safety and prevents atmospheric contamination |
| High-Transmittance Lens | Minimizes energy loss/attenuation | Ensures consistent heating for complete synthesis |
Elevate Your Advanced Material Synthesis with KINTEK
Achieving phase-pure Hafnium Carbide requires uncompromising atmospheric control and precision energy delivery. At KINTEK, we understand the complexities of Selective Laser Reactive Pyrolysis. Backed by expert R&D and world-class manufacturing, we provide high-performance Vacuum systems, CVD systems, and customizable lab high-temperature furnaces designed to meet your specific research demands.
Whether you need an inert environmental chamber or a specialized high-temp furnace tailored for unique laser-integrated applications, our technical team is ready to assist. Contact us today to optimize your lab's synthesis capabilities!
Visual Guide
References
- Shalini Rajpoot, Chengying Xu. Synthesis of hafnium carbide (HfC) via one‐step selective laser reaction pyrolysis from liquid polymer precursor. DOI: 10.1111/jace.20650
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does industrial-scale forging equipment influence the morphology of primary carbonitrides in H13 tool steel?
- How do high-temp furnaces influence LTO sintering? Optimize Lithium Titanate Performance via Precision Control
- How does the lab oven drying process ensure the quality of bimetallic catalysts? Master Pore Stability & Dispersion
- What are the advantages of using a programmable high-temperature laboratory furnace for CSA cement? Precision Control
- What is the function of a forced drying oven in SiOC coating conversion? Ensure Flawless Solvent Removal
- How does a continuous argon flow heating chamber aid CMF testing? Ensure Pure Thermal Analysis
- What is the main benefit of using a benchtop industrial oven? Save Space and Boost Efficiency in Your Lab
- What role does Thermogravimetric Analysis (TGA) play in determining the calcination parameters for manganese phosphate?



















