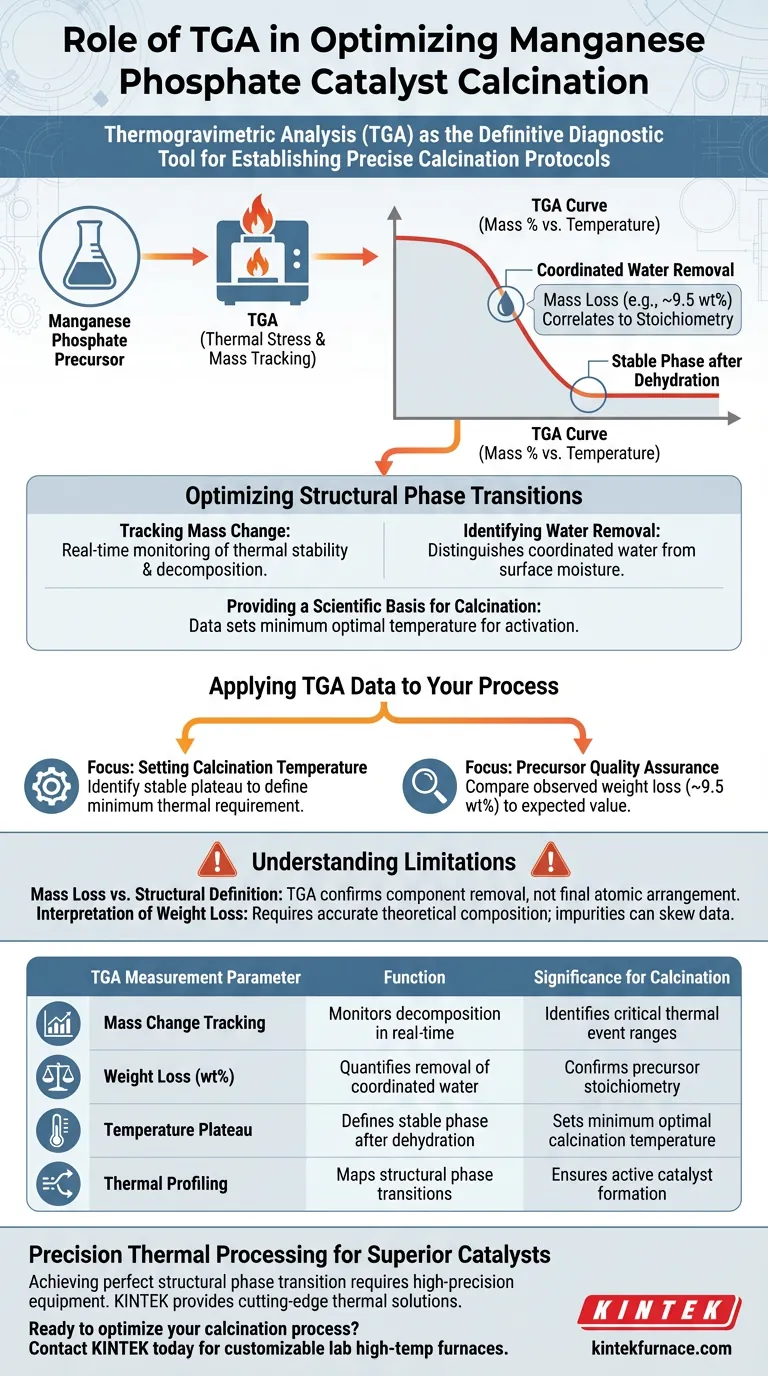
Thermogravimetric Analysis (TGA) serves as the definitive diagnostic tool for establishing precise calcination protocols by tracking mass changes under thermal stress. Specifically, it identifies the exact temperature required to remove coordinated water from manganese phosphate precursors, providing the data necessary to set the optimal temperature for activation.
By quantifying the specific weight loss associated with water removal, TGA transforms calcination from an estimation into a calculated process, ensuring the precursor undergoes the necessary structural phase transitions to become an active catalyst.

The Science of Thermal Profiling
Tracking Mass Change
TGA continuously records the mass of the manganese phosphate sample as it undergoes a controlled temperature ramp. This real-time monitoring is critical for visualizing the sample's thermal stability and decomposition behavior. By observing where the mass drops, you can pinpoint the exact thermal events relevant to catalyst preparation.
Identifying Coordinated Water Removal
The primary function of TGA in this context is to determine the removal temperature of coordinated water. Unlike surface moisture, coordinated water is chemically bound to the crystal structure. TGA distinguishes this event, allowing you to identify the specific temperature range where these bonds break and the water molecules are released.
Optimizing Structural Phase Transitions
Correlating Weight Loss to Chemistry
TGA provides a quantitative method to verify the stoichiometry of your precursor. By analyzing the magnitude of the weight loss, you can confirm if it matches the theoretical expectations for the material. For manganese phosphate precursors, a weight loss of approximately 9.5 wt% serves as a specific marker, confirming the correct loss of coordinated water molecules.
Providing a Scientific Basis for Calcination
The data derived from TGA acts as the foundational evidence for your thermal treatment parameters. To induce the desired structural phase transitions, the calcination temperature must be set based on the completion of the dehydration process observed in the TGA curve. This ensures the catalyst structure is fully evolved without overheating the material.
Understanding the Limitations
Mass Loss vs. Structural Definition
While TGA is excellent for determining when a transformation occurs based on mass, it does not explicitly show the final atomic arrangement. It confirms the removal of components (like water) necessary for a phase transition, but it does not visualize the resulting crystal lattice.
Interpretation of Weight Loss
Reliance on weight loss data requires accurate knowledge of the precursor's theoretical composition. If the precursor contains impurities or unexpected solvates, the weight loss percentage (e.g., the target 9.5 wt%) may be misinterpreted, leading to incorrect calcination assumptions.
Applying TGA Data to Your Process
If your primary focus is setting the calcination temperature:
- Identify the temperature on the TGA curve where the mass loss stabilizes (plateaus) after the coordinated water removal event to define your minimum thermal requirement.
If your primary focus is precursor quality assurance:
- Compare the observed weight loss against the expected value (such as ~9.5 wt%) to verify that the precursor has the correct chemical composition before investing energy in calcination.
Precise thermal analysis ensures that your energy input yields the correct structural phase for maximum catalytic performance.
Summary Table:
| TGA Measurement Parameter | Function in Catalyst Preparation | Significance for Calcination |
|---|---|---|
| Mass Change Tracking | Monitors decomposition in real-time | Identifies critical thermal event ranges |
| Weight Loss (wt%) | Quantifies removal of coordinated water | Confirms precursor stoichiometry (e.g., ~9.5 wt%) |
| Temperature Plateau | Defines stable phase after dehydration | Sets the minimum optimal calcination temperature |
| Thermal Profiling | Maps structural phase transitions | Ensures active catalyst formation without overheating |
Precision Thermal Processing for Superior Catalysts
Achieving the perfect structural phase transition in manganese phosphate catalysts requires more than just data—it requires high-precision equipment. KINTEK provides the cutting-edge thermal solutions necessary to translate your TGA findings into scalable results.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are refining catalyst activation or performing complex material synthesis, our lab high-temp furnaces are fully customizable to meet your unique research needs.
Ready to optimize your calcination process? Contact KINTEK today to consult with our experts and find the ideal furnace for your laboratory.
Visual Guide
References
- Shujiao Yang, Wei Zhang. Electrocatalytic water oxidation with manganese phosphates. DOI: 10.1038/s41467-024-45705-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Ultra High Vacuum CF Flange Stainless Steel Sapphire Glass Observation Sight Window
People Also Ask
- Why is a constant temperature drying oven utilized at 40 °C for clayey raw materials? Ensure Mineral Integrity.
- What is the function of a furnace in CuAlMn alloy treatment? Achieve Perfect Microstructural Homogenization
- What additional benefits do vacuum chambers provide beyond environmental control? Enhance Material Purity and Process Efficiency
- Why is a stainless steel high-pressure autoclave essential for starch hydrogenation? Unlock Peak Reaction Efficiency
- Why is it necessary to dry glassware in a 140 °C oven overnight before GTP? Ensure Precise Anhydrous Polymerization
- What are the primary functions of high-purity nitrogen flow in carbon pyrolysis? Optimize Purity and Pore Structure
- What are the advantages of using a vacuum drying oven for BiVO4/COF composite photoanodes? Preserve Material Integrity
- How are thermal processing equipment commonly categorized? Choose the Right Furnace for Your Lab

