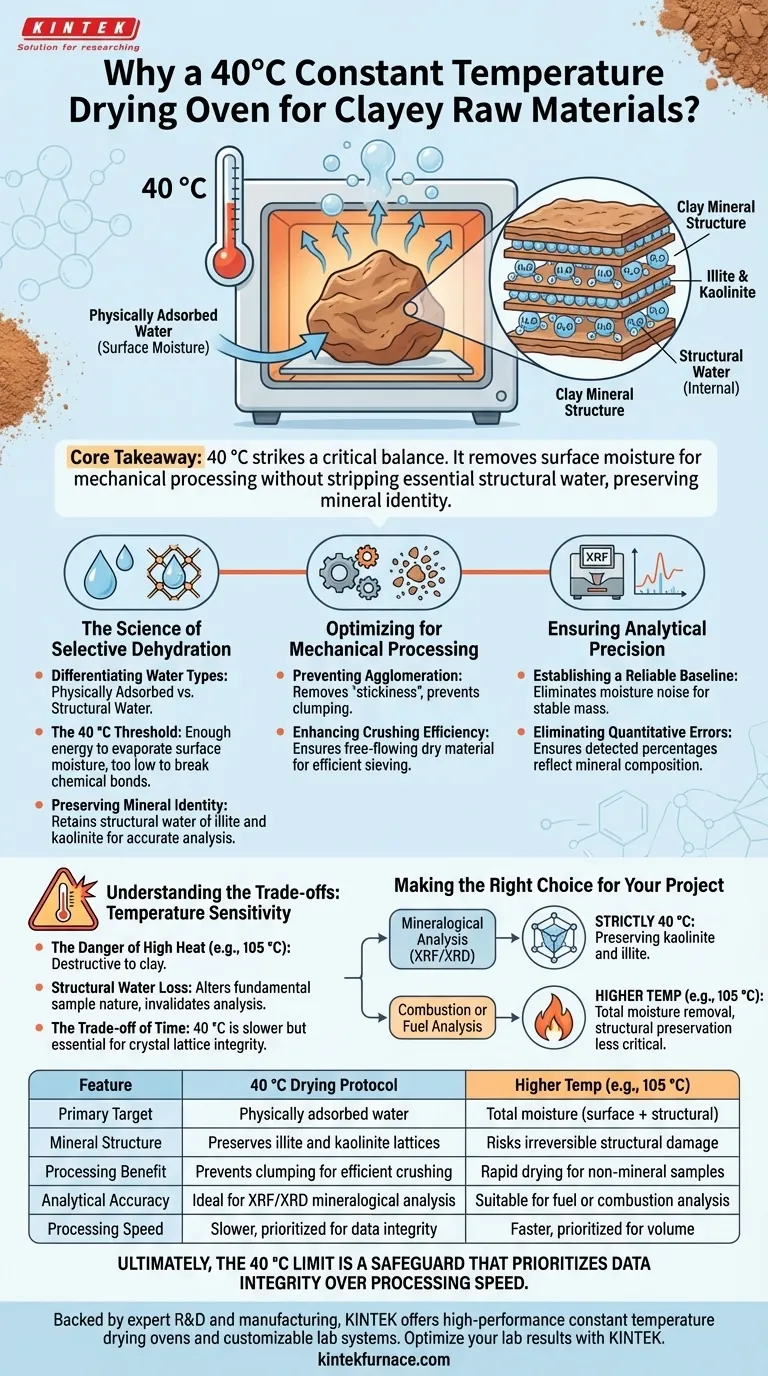
The primary function of utilizing a constant temperature drying oven at 40 °C is to selectively remove physically adsorbed water from clayey raw materials while strictly preserving their internal chemical structure. By maintaining this specific low-temperature environment, you ensure the material becomes dry enough for mechanical processing without stripping away the structural water essential to minerals like illite and kaolinite.
Core Takeaway Processing clay at 40 °C strikes a critical balance between dehydration and mineral preservation. It eliminates surface moisture to facilitate crushing and accurate chemical analysis, yet remains cool enough to prevent the irreversible alteration of the clay's crystallographic structure.

The Science of Selective Dehydration
Differentiating Water Types
In clay mineralogy, not all water is the same. You are dealing with two distinct types: physically adsorbed water (surface moisture) and structural water (part of the crystal lattice).
The 40 °C Threshold
A temperature of 40 °C is specifically chosen because it provides enough energy to evaporate surface moisture but is too low to break the chemical bonds holding structural water.
Preserving Mineral Identity
If temperatures exceed this threshold, you risk damaging clay minerals such as illite and kaolinite. Retaining their structural water is vital for accurate characterization later in the workflow.
Optimizing for Mechanical Processing
Preventing Agglomeration
Wet clay naturally adheres to itself and machinery. Drying at 40 °C removes the "stickiness" caused by adsorbed water.
Enhancing Crushing Efficiency
This dehydration step prevents mineral clumping. By ensuring the raw material is physically dry, subsequent crushing and sieving processes become significantly more efficient and uniform.
Ensuring Analytical Precision
Establishing a Reliable Baseline
For chemical composition analysis, particularly X-ray Fluorescence (XRF), moisture creates noise in the data. Water content fluctuates with humidity, making wet samples unreliable standards.
Eliminating Quantitative Errors
By removing adsorbed water, you stabilize the sample's mass. This eliminates quantitative errors in the final data, ensuring that the percentages detected reflect the mineral composition, not the water weight.
Understanding the Trade-offs: Temperature Sensitivity
The Danger of High Heat
It is a common error to assume that "hotter is better" for drying. While fuels may be dried at 105 °C to remove all moisture completely, applying this temperature to clay can be destructive.
Structural Water Loss
At temperatures like 105 °C, clay minerals may begin to lose their structural water. This alters the fundamental nature of the sample, rendering subsequent mineralogical analysis invalid.
The Trade-off of Time
The trade-off for using the safer 40 °C limit is time. It is a slower process than high-heat drying, but it is the only way to ensure the integrity of the clay's crystal lattice is maintained.
Making the Right Choice for Your Project
To determine the correct drying protocol, you must align your method with your specific analytical goals:
- If your primary focus is Mineralogical Analysis (XRF/XRD): Stick strictly to 40 °C. Preserving the structural water of kaolinite and illite is non-negotiable for accurate identification.
- If your primary focus is Combustion or Fuel Analysis: You may require higher temperatures (e.g., 105 °C) to eliminate all endothermic moisture effects, as structural preservation is less critical than total moisture removal.
Ultimately, the 40 °C limit is a safeguard that prioritizes data integrity over processing speed.
Summary Table:
| Feature | 40 °C Drying Protocol | Higher Temp (e.g., 105 °C) |
|---|---|---|
| Primary Target | Physically adsorbed water (surface moisture) | Total moisture (surface + structural) |
| Mineral Structure | Preserves illite and kaolinite lattices | Risks irreversible structural damage |
| Processing Benefit | Prevents clumping for efficient crushing | Rapid drying for non-mineral samples |
| Analytical Accuracy | Ideal for XRF/XRD mineralogical analysis | Suitable for fuel or combustion analysis |
| Processing Speed | Slower, prioritized for data integrity | Faster, prioritized for volume |
Precision is paramount in clay mineralogy. Backed by expert R&D and manufacturing, KINTEK offers high-performance constant temperature drying ovens and customizable lab systems tailored for sensitive material processing. Whether you need Muffle, Tube, or Vacuum furnaces, our equipment ensures your samples maintain their structural integrity for accurate XRF and XRD analysis. Optimize your lab results with KINTEK—contact us today!
Visual Guide
References
- Carla Candeias, Fernando Rocha. Clay Schists from Barrancos (Portugal): An Approach Toward Sustainable Ceramic Raw Material Use. DOI: 10.3390/min15080852
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the function of a constant temperature blast drying oven? Achieve Uniform Chemical Activation and Porosity
- How does industrial-scale forging equipment influence the morphology of primary carbonitrides in H13 tool steel?
- How does a high-temperature annealing furnace regulate cold-rolled steel? Optimize Manganese Steel Performance
- How does a Bridgman furnace control single-crystal quality? Master Precision Directional Solidification
- How does a circulating mineral oil jacket heating system function? Ensure Precision in Wood Thermal Modification
- What is the function of a sintering aid reservoir? Unlock Rapid Densification via MV-Sintering Technology
- What is the purpose of using high-purity argon gas for NAB alloys? Ensure Superior Nickel-Aluminum Bronze Integrity
- How do regenerative burners enhance the energy efficiency of billet heating furnaces? Boost Performance by 50%



















