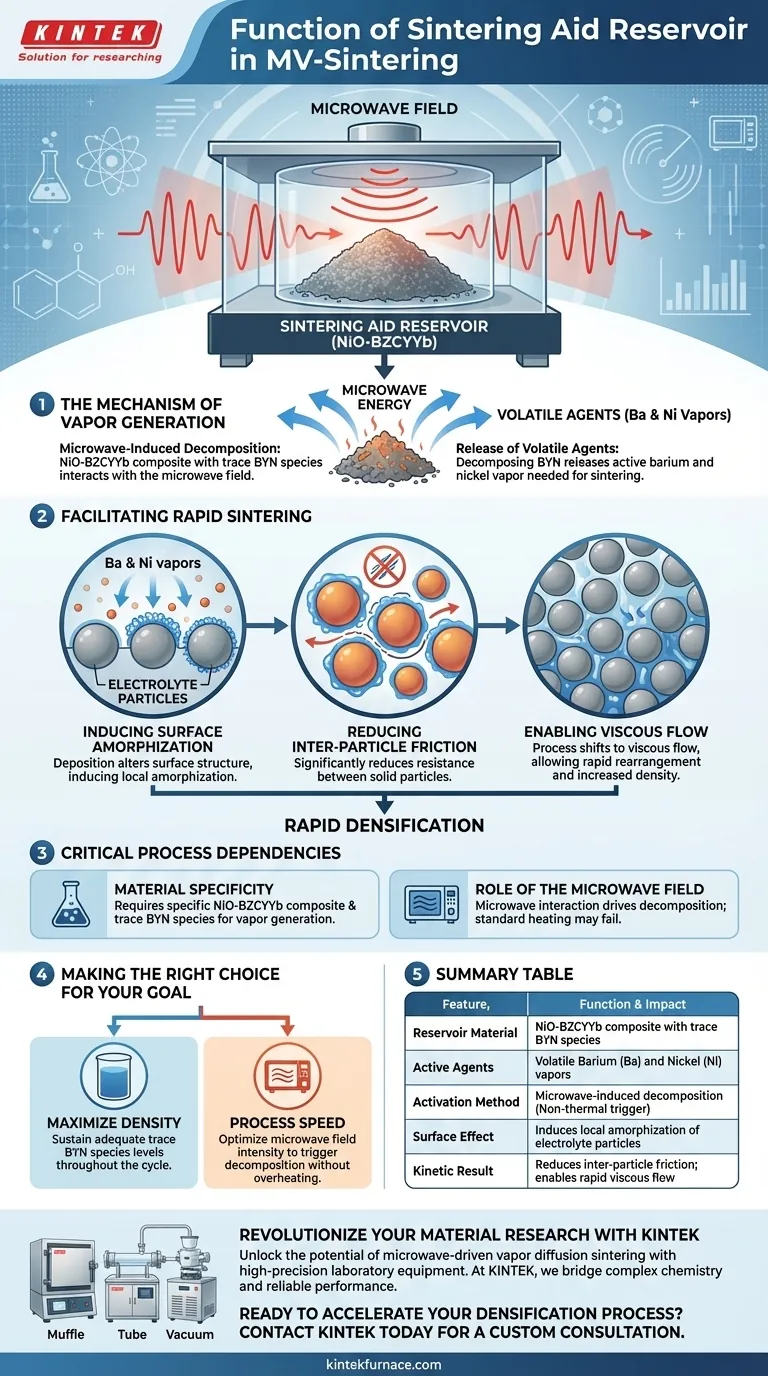
The primary function of the sintering aid reservoir in microwave-driven vapor diffusion sintering (MV-sintering) is to act as a dynamic source of volatile chemical species that catalyze the densification of electrolyte particles. Constructed from NiO-BZCYYb composite materials, the reservoir decomposes under microwave irradiation to release essential vapors that modify particle surfaces and reduce friction.
Core Takeaway The reservoir utilizes microwave energy to transform solid precursors into an active vapor containing barium and nickel. This vapor coats the target particles, inducing a "viscous flow" state that allows the material to densify significantly faster than it would through thermal energy alone.

The Mechanism of Vapor Generation
Microwave-Induced Decomposition
The reservoir is composed of NiO-BZCYYb composite materials which contain trace BYN species. Unlike a passive container, this material interacts directly with the microwave field. This interaction triggers the decomposition of the trace species within the reservoir.
Release of Volatile Agents
As the BYN species decompose, they release a vapor media specifically containing barium and nickel. These are not inert byproducts; they are the active agents required for the sintering process. Once released, these species diffuse from the reservoir to the electrolyte particles being processed.
Facilitating Rapid Sintering
Inducing Surface Amorphization
When the barium and nickel vapors deposit onto the electrolyte particles, they alter the material's surface structure. This deposition induces local amorphization, meaning the crystalline structure at the particle surface becomes disordered or glass-like.
Reducing Inter-Particle Friction
The physical consequence of this amorphization is a significant reduction in inter-particle friction. The solid particles are no longer grinding against one another with high resistance.
Enabling Viscous Flow
With friction reduced, the process shifts to a viscous flow mechanism. This allows the particles to slide and rearrange rapidly, filling gaps and increasing density much more efficiently than traditional solid-state diffusion.
Critical Process Dependencies
Material Specificity
The success of this process is strictly tied to the chemical composition of the reservoir. Without the specific NiO-BZCYYb composite and its trace BYN species, the necessary barium and nickel vapors will not be generated.
The Role of the Microwave Field
The reservoir functions only under the influence of a microwave field. It is the specific interaction between the microwaves and the trace species that drives the decomposition; standard thermal heating may not trigger the release of these specific volatile agents.
Making the Right Choice for Your Goal
To optimize the MV-sintering process, you must view the reservoir not just as a tool, but as a chemical reagent that dictates the speed of your results.
- If your primary focus is maximizing density: Ensure your reservoir material maintains adequate levels of trace BYN species to sustain the vapor supply throughout the cycle.
- If your primary focus is process speed: Optimize the microwave field intensity to sufficiently trigger the decomposition of the reservoir material without overheating the target electrolyte.
By precise control of the reservoir's activation, you leverage vapor diffusion to achieve rapid, high-quality material consolidation.
Summary Table:
| Feature | Function & Impact |
|---|---|
| Reservoir Material | NiO-BZCYYb composite containing trace BYN species |
| Active Agents | Volatile Barium (Ba) and Nickel (Ni) vapors |
| Activation Method | Microwave-induced decomposition (Non-thermal trigger) |
| Surface Effect | Induces local amorphization of electrolyte particles |
| Kinetic Result | Reduces inter-particle friction; enables rapid viscous flow |
Revolutionize Your Material Research with KINTEK
Unlock the full potential of microwave-driven vapor diffusion sintering with high-precision laboratory equipment. At KINTEK, we bridge the gap between complex sintering chemistry and reliable performance.
Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which can be customized to meet your specific research or industrial needs. Whether you are optimizing electrolyte density or exploring advanced vapor-phase catalysis, our technical team is ready to help you engineer the perfect thermal environment.
Ready to accelerate your densification process?
Contact KINTEK today for a custom consultation
Visual Guide
References
- Dongyeon Kim, Kang Taek Lee. Sub‐1000 °C Sintering of Protonic Ceramic Electrochemical Cells via Microwave‐Driven Vapor Phase Diffusion. DOI: 10.1002/adma.202506905
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does a precision mass loss measurement system play? Identifying Vapor Pressure in High-Temp Furnaces
- Why is precise heating rate control necessary? Master Activated Carbon Heat Treatment with KINTEK
- What is the purpose of using a high-vacuum pump system for NiTi thin films? Ensure Pure Stoichiometry & Performance
- What is the significance of using a laboratory electric furnace for the quenching and tempering of hull steel? Achieve Precise Microstructure Control
- Why is an 800 °C heat treatment for Ti6Al4V additive manufacturing necessary? Unlock Ductility & Relieve Stress
- What is the primary role of an industrial-grade oven in the preparation of chitosan-modified soil samples?
- What role does an RTA system play in processing SiN thin films? Unlock High-Performance Quantum & Optical Materials
- What role does pack media play in the solid-state powder boriding process? Enhance Metal Hardness at High Temperatures



















