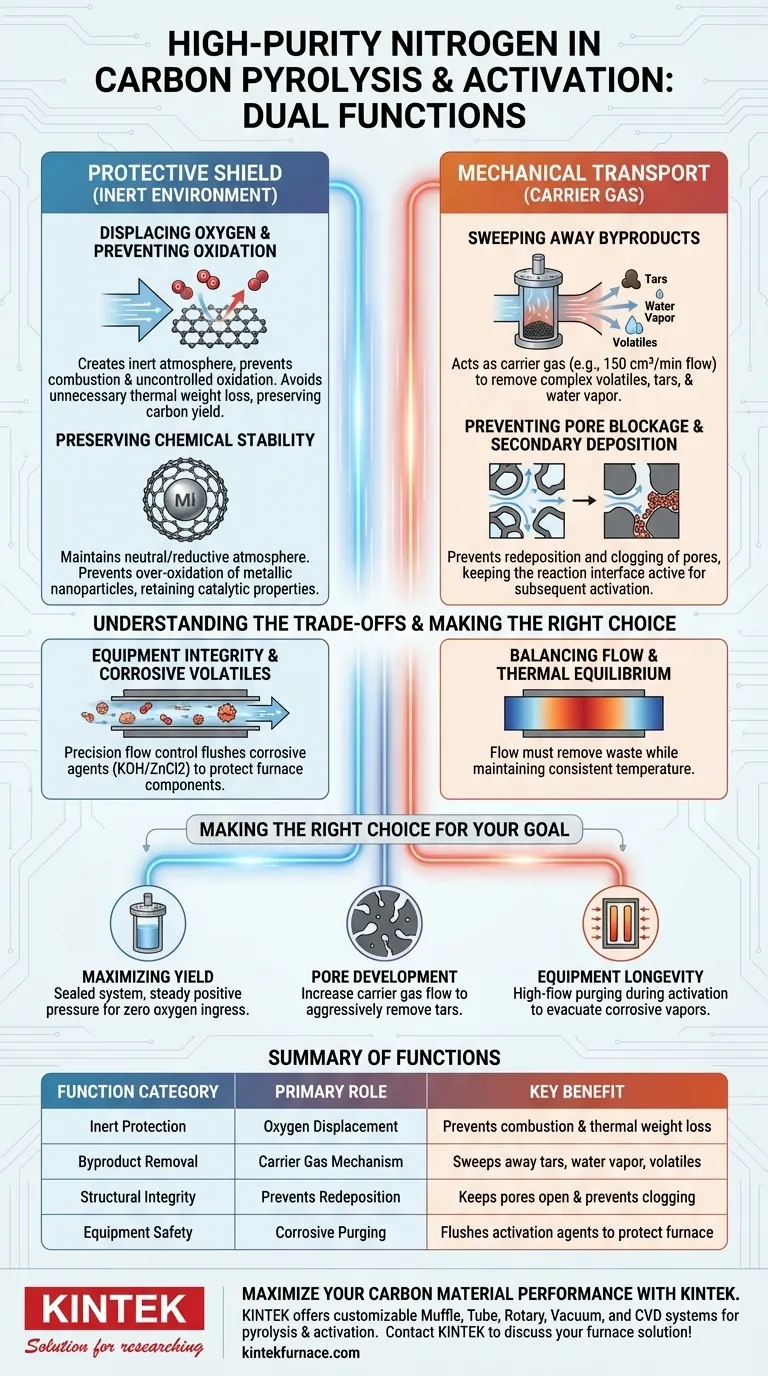
High-purity nitrogen serves two dual-purpose functions in the processing of carbon materials: it acts as a protective shield against chemical degradation and a mechanical transport system for waste. Primarily, it creates an inert environment by displacing oxygen to prevent the carbon from burning or oxidizing uncontrollably. Simultaneously, it functions as a carrier gas to actively sweep away tar, water vapor, and volatile decomposition products that would otherwise clog the material's pores.
The success of carbon pyrolysis relies on nitrogen’s ability to maintain a strictly inert atmosphere while continuously purging byproducts, thereby preserving both the material's mass and its developing pore structure.

Creating a Stable Reaction Environment
Inert Protection Against Oxidation
The most immediate function of nitrogen flow is the physical displacement of oxygen within the reactor. Without this exclusion of oxygen, the high temperatures required for pyrolysis would cause the carbon material to combust.
By replacing the air with nitrogen, you prevent unnecessary thermal weight loss. This ensures that the reduction in mass is due to the desired release of volatiles, not the destruction of your carbon yield.
Preserving Chemical Stability
Beyond basic combustion prevention, nitrogen establishes a stable neutral or reductive atmosphere. This is critical for maintaining the chemical stability of the carbon support itself.
If your material contains metallic nanoparticles, this inert blanket prevents their over-oxidation. This ensures that any catalytic properties or specific chemical functionalities are preserved during the thermal treatment.
Managing Decomposition Byproducts
The Carrier Gas Mechanism
During pyrolysis, the carbon precursor breaks down, releasing complex volatiles, tars, and water vapor. Nitrogen acts as a generic "carrier gas," physically transporting these substances out of the hot zone.
Effective removal often requires specific flow rates (e.g., 150 cm³/min) to ensure adequate velocity. This constant movement prevents the reactor atmosphere from becoming saturated with waste products.
Preventing Pore Blockage and Secondary Deposition
If volatiles are allowed to linger in the reactor, they can redeposit onto the carbon surface or decompose further. This leads to secondary deposition, which can seal off the very pores you are trying to create.
By continuously purging these byproducts, nitrogen maintains the activity of the reaction interface. This keeps the pore structure open and accessible for subsequent activation or final application.
Understanding the Trade-offs
Equipment Integrity and Corrosive Volatiles
During chemical activation (using agents like KOH or ZnCl2), the process releases corrosive volatiles. An insufficient nitrogen flow doesn't just harm the sample; it endangers your equipment.
A precision flow control system is necessary to flush these corrosive elements out of the tube furnace. This protects the internal components of your heating elements and sensors from rapid degradation.
Balancing Flow and Thermal Equilibrium
While flow is essential, it must be balanced to maintain chemical equilibrium. The flow must be sufficient to remove waste but stable enough to ensure consistent temperature distribution across the sample.
Making the Right Choice for Your Goal
To optimize your pyrolysis or activation process, tailor your nitrogen flow strategy to your specific outcome:
- If your primary focus is Maximizing Yield: Prioritize a rigorously sealed system with steady nitrogen positive pressure to ensure zero oxygen ingress and minimal carbon burn-off.
- If your primary focus is Pore Development: Increase the carrier gas flow rate to aggressively remove tars and volatiles, preventing them from blocking micropores.
- If your primary focus is Equipment Longevity: Ensure high-flow purging during chemical activation steps to rapidly evacuate corrosive byproducts like potassium or zinc vapors.
Mastering the nitrogen flow is not just about safety; it is the control knob for defining the final texture and purity of your carbon material.
Summary Table:
| Function Category | Primary Role | Key Benefit |
|---|---|---|
| Inert Protection | Oxygen Displacement | Prevents combustion and unnecessary thermal weight loss |
| Byproduct Removal | Carrier Gas Mechanism | Sweeps away tars, water vapor, and volatiles |
| Structural Integrity | Prevents Redeposition | Keeps pores open and prevents secondary carbon deposition |
| Equipment Safety | Corrosive Purging | Flushes activation agents (KOH/ZnCl2) to protect furnace components |
Maximize Your Carbon Material Performance with KINTEK
Precision thermal processing is the key to achieving superior carbon yields and optimized pore structures. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique pyrolysis and activation requirements.
Whether you are focusing on maximizing yield or developing complex micropores, our lab high-temp furnaces provide the stable atmosphere and flow control you need for consistent results.
Ready to elevate your lab's capabilities? Contact KINTEK today to discuss your customized furnace solution!
Visual Guide
References
- Aik Chong Lua. Conversion of Oil Palm Kernel Shell Wastes into Active Biocarbons by N2 Pyrolysis and CO2 Activation. DOI: 10.3390/cleantechnol7030066
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Spark Plasma Sintering SPS Furnace
People Also Ask
- What is the function of a drying oven in the chemical activation of biochar with phosphoric acid? Optimize Biochar Quality
- What is the role of sintering in CsPbBr3-SiO2 preparation? Unlock Ultra-Stability with Precise Thermal Sealing
- How does the availability of specialized furnace systems benefit chemical research? Optimize Your Thermal Processing
- How does a temperature-programmed system influence molybdenum carbide formation? Expert Catalyst Synthesis Guide
- What additional techniques are used in activated sintering? Boost Efficiency with Advanced Chemical Methods
- What hardware characteristics are required for a reactor system to support a three-step redox process in chemical looping?
- How do thermal systems reveal anti-spalling mechanisms in CDE concrete? Explore Advanced Material Resilience
- What is the purpose of using a liquid nitrogen adsorption instrument and BET analysis? Characterize RCM Nanosheets








