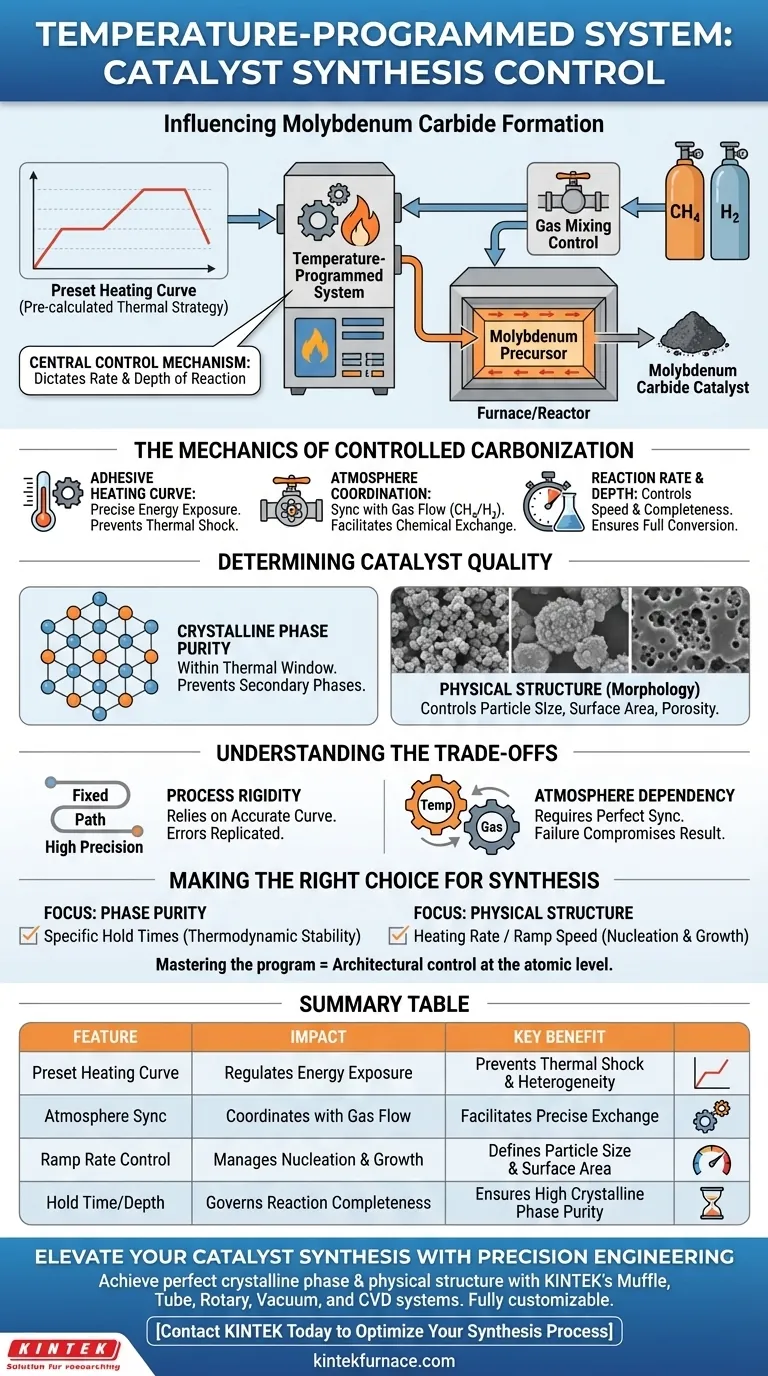
A temperature-programmed system serves as the central control mechanism for synthesizing molybdenum carbide. By strictly adhering to a preset heating curve within a specific carbonization atmosphere—typically a methane/hydrogen mixture—this system dictates the precise rate and depth of the reaction, directly determining the catalyst's final properties.
The core function of this system is to replace variable heating conditions with a rigorous, reproducible thermal profile. This precision ensures that the transformation from precursor to carbide results in specific crystalline phases and physical structures, rather than a random mixture of byproducts.

The Mechanics of Controlled Carbonization
Adhering to the Preset Heating Curve
The system does not simply apply heat; it executes a pre-calculated thermal strategy.
By following a specific heating curve, the system ensures the material is exposed to the exact energy levels required at every second of the process. This prevents thermal shock or uneven heating that could lead to heterogeneous samples.
Coordinating with Gas Atmosphere
Temperature control does not happen in a vacuum; it works in tandem with gas mixing control devices.
The reference highlights that the heating curve operates within a specific carbonization atmosphere, such as a methane and hydrogen mixture. The temperature program must align with the gas flow to facilitate the correct chemical exchange between the solid precursor and the gas phase.
Controlling Reaction Rate and Depth
The primary variable influenced by the temperature program is the reaction kinetics.
By modulating how fast the temperature rises and how long it holds, the system controls the speed (rate) and completeness (depth) of the carbonization. This control is the difference between a fully converted catalyst and one with an unreacted core.
Determining Catalyst Quality
Regulating Crystalline Phase Purity
The specific arrangement of atoms—the crystalline phase—is highly sensitive to temperature.
The temperature-programmed system ensures the synthesis remains within the thermal window required for the desired phase. This prevents the formation of unwanted secondary phases that would dilute the purity of the molybdenum carbide.
Defining Physical Structure
Beyond chemistry, the thermal profile dictates the morphology of the catalyst.
The reference notes that this control is the "core method" for regulating physical structure. This implies that factors such as particle size, surface area, and porosity are outcomes of how the temperature program manages the sintering and reaction rates.
Understanding the Trade-offs
Process Rigidity
A temperature-programmed system relies heavily on the accuracy of the preset curve.
Because the system follows a fixed path, any error in the initial programming or calculation of the curve will be replicated perfectly in the final product. The system offers high precision but requires significant upfront optimization to define the correct parameters.
Atmosphere Dependency
Success is not defined by temperature alone; it is dependent on the gas mixture stability.
Even with a perfect heating curve, if the gas mixing devices fail to maintain the correct methane/hydrogen ratio, the temperature program cannot compensate. The two systems must operate in perfect synchronization.
Making the Right Choice for Your Synthesis
To optimize your molybdenum carbide synthesis, consider which parameter is most critical for your application:
- If your primary focus is Phase Purity: Ensure your heating curve includes specific hold times at temperatures that favor the thermodynamic stability of the desired crystal phase.
- If your primary focus is Physical Structure: Prioritize the heating rate (ramp speed) to control nucleation and growth, preventing excessive sintering that reduces surface area.
Mastering the temperature program is not just about heating a sample; it is about architectural control over the material at the atomic level.
Summary Table:
| Feature | Impact on Synthesis | Key Benefit |
|---|---|---|
| Preset Heating Curve | Regulates energy exposure levels | Prevents thermal shock & heterogeneity |
| Atmosphere Sync | Coordinates with $CH_4/H_2$ gas flow | Facilitates precise solid-gas chemical exchange |
| Ramp Rate Control | Manages nucleation and growth speeds | Defines particle size and surface area |
| Hold Time/Depth | Governs reaction completeness | Ensures high crystalline phase purity |
Elevate Your Catalyst Synthesis with Precision Engineering
Achieving the perfect crystalline phase and physical structure in molybdenum carbide requires absolute thermal control. KINTEK provides the cutting-edge technology needed to master these variables. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique temperature-programmed synthesis requirements.
Don't let inconsistent heating compromise your research. Partner with KINTEK for lab high-temp furnaces that deliver rigorous, reproducible results.
Contact KINTEK Today to Optimize Your Synthesis Process
Visual Guide
References
- Ying Yang, Kunyu Xu. Controllable synthesis of transition metal-modified molybdenum carbide crystalline phases and its application on hydrodeoxygenation of phenol. DOI: 10.1051/e3sconf/202562501016
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What are the advantages of electric current-assisted TLP bonding? Maximize Efficiency for Inconel 718 Joining
- What role does precise temperature control play in nuclear waste leaching tests? Ensure Accurate Safety Evaluations
- Why do high-performance Bi-2223 superconducting materials require high-precision temperature control? | KINTEK Solution
- What are the primary objectives of using a blast drying oven for In2O3/C nanofibers? Ensure Structural Integrity
- What is sintering and what types of materials can it be applied to? Unlock Dense, Strong Materials for Your Projects
- What are the benefits of using electric actuators in this solution? Achieve Precision, Safety, and Efficiency in Automation
- What is the primary purpose of drying and calcination in nickel laterite ore pretreatment? Optimize Your Smelting Efficiency
- What is the primary role of a ball mill in raw material preparation for vacuum carbothermic reduction of magnesium? Ensure a Complete and Rapid Reaction


















