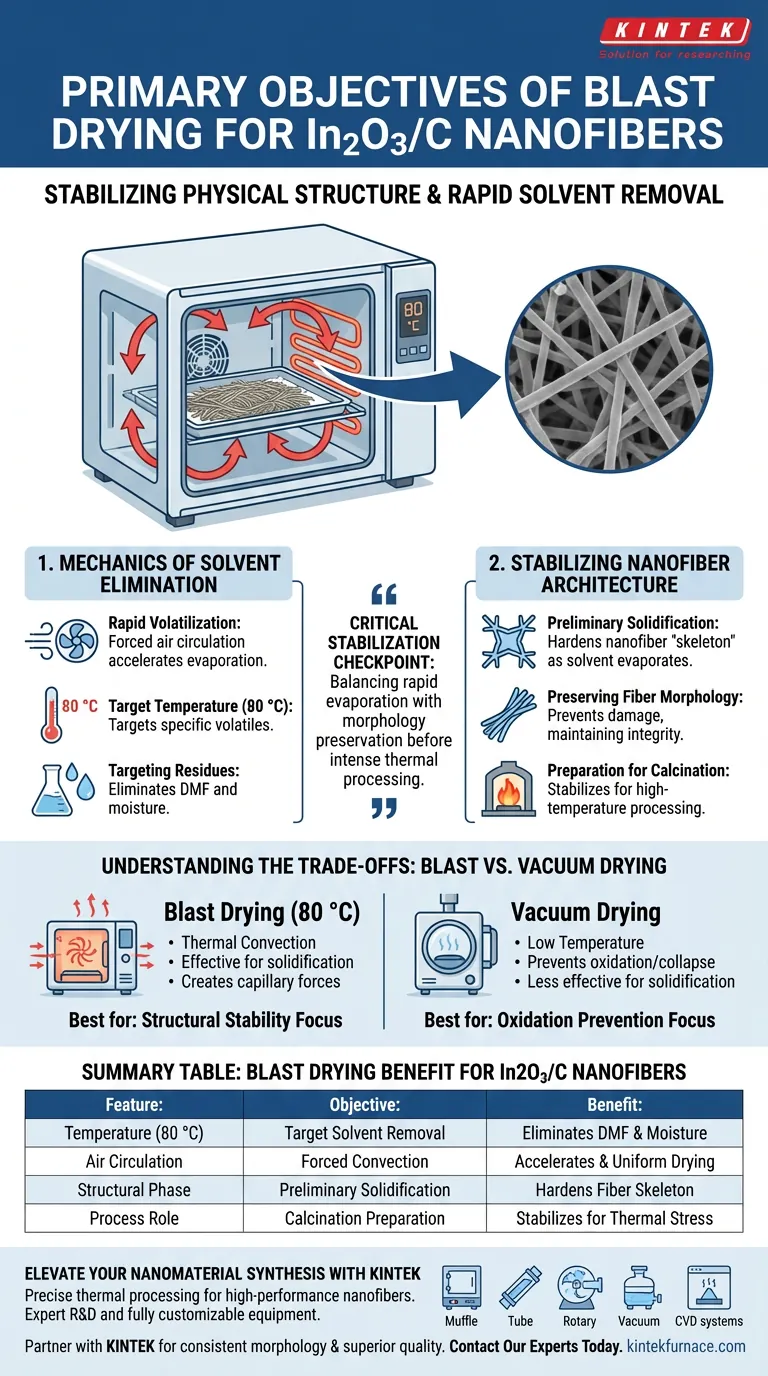
The primary objective of using a blast drying oven for In2O3/C nanofibers is to stabilize the material's physical structure through the rapid removal of volatile components. Typically operating at 80 °C, this heat treatment eliminates residual organic solvents, such as N,N-Dimethylformamide (DMF), and adsorbed moisture from the as-spun fiber mats. Crucially, this process achieves preliminary solidification of the nanofiber skeleton, ensuring the morphology remains intact prior to high-temperature calcination.
The blast drying stage serves as a critical stabilization checkpoint, balancing the need for rapid solvent evaporation with the necessity of preserving the delicate nanofiber geometry before the material undergoes intense thermal processing.
The Mechanics of Solvent Elimination
Rapid Volatilization
The blast drying oven utilizes forced air circulation to accelerate the evaporation process.
By maintaining a consistent temperature of 80 °C, the oven targets specific volatile components remaining from the electrospinning process.
Targeting Specific Residues
The primary targets during this phase are residual organic solvents, specifically DMF, and any moisture adsorbed from the environment.
Removing these impurities is essential to prevent structural defects or uncontrolled reactions during subsequent heating stages.
Stabilizing the Nanofiber Architecture
Preliminary Solidification
Beyond simple drying, this step acts as a hardening phase for the nanofiber "skeleton."
As the solvent evaporates, the polymer-precursor matrix solidifies, locking the fibers into their as-spun arrangement.
Preserving Fiber Morphology
The temperature control provided by the blast oven is precise enough to dry the material without degrading it.
This ensures that the fiber morphology is not damaged or distorted, maintaining the surface area and structural integrity required for the final product.
Preparation for Calcination
This drying phase is a prerequisite for the high-temperature calcination process.
By removing volatiles and solidifying the structure beforehand, the material is mechanically prepared to withstand the thermal stresses of carbonization and crystallization that follow.
Understanding the Trade-offs
Blast Drying vs. Vacuum Drying
While a blast drying oven is effective for solidifying In2O3/C skeletons, it relies on thermal convection and higher temperatures (80 °C).
In contrast, vacuum drying is often used for materials that are highly sensitive to oxidation or capillary collapse, such as MoSe2 nanosheets.
Risk of Structural Collapse
Blast drying is efficient, but creates capillary forces during evaporation that could theoretically damage extremely fragile structures.
However, for In2O3/C nanofibers, the blast oven strikes the right balance: it provides the necessary heat for solidification that vacuum drying (which typically operates at lower temperatures to prevent phase transformations) might not achieve as effectively for this specific precursor.
Optimizing the Drying Strategy
To ensure high-quality nanofiber synthesis, align your drying method with your structural goals.
- If your primary focus is Structural Stability: Prioritize the blast drying oven at 80 °C to achieve rapid solvent removal and the necessary solidification of the In2O3/C skeleton.
- If your primary focus is Oxidation Prevention: Verify the sensitivity of your specific precursor; if the material is prone to phase transformation or pore collapse at 80 °C, a vacuum approach might be required, though it is less standard for this specific nanofiber type.
Successful drying solidifies the precursor's physical foundation, ensuring the final calcined material retains the desired nanofiber morphology.
Summary Table:
| Feature | Blast Drying Objective | Benefit for In2O3/C Nanofibers |
|---|---|---|
| Temperature (80 °C) | Target Solvent Removal | Eliminates DMF and moisture effectively |
| Air Circulation | Forced Convection | Accelerates evaporation and ensures uniform drying |
| Structural Phase | Preliminary Solidification | Hardens fiber skeleton to prevent morphology collapse |
| Process Role | Calcination Preparation | Stabilizes material for high-temperature thermal stress |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise thermal processing is the foundation of high-performance nanofiber production. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of laboratory solutions, including high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems.
Whether you need to stabilize In2O3/C skeletons or execute complex calcination, our equipment is fully customizable to meet your unique research and industrial needs. Partner with KINTEK to ensure consistent morphology and superior material quality.
Contact Our Experts Today to find the perfect thermal solution for your lab.
Visual Guide
References
- Wenhe Xie, Xiaolei Sun. Encapsulating Ultrafine In2O3 Particles in Carbon Nanofiber Framework as Superior Electrode for Lithium-Ion Batteries. DOI: 10.3390/inorganics12120336
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a high-precision furnace required for carbon aerogel activation? Achieve Optimal Pore Development & Control
- How do aerospace industries benefit from high-temperature furnaces? Unlock Superior Strength and Durability
- How does the analysis of optimized process paths assist in lab equipment selection? Expert Guide for Research Success
- Why is a vacuum drying oven used for BC-FeOOH biochar? Protect Reactivity and Prevent Particle Aggregation
- What is the function of planetary ball mills or industrial mixing granulators prior to RHF? Optimize FMDS Reactivity
- What is the purpose of the sulfidation treatment process? Enhance Reactor Performance with DMDS Passivation
- What role does temperature control play in biomass pyrolysis for biochar? Achieve Optimal Pore Structure & Yield
- Why is temperature gradient management necessary for high-temperature impedance measurements? Master Thermal Precision



















