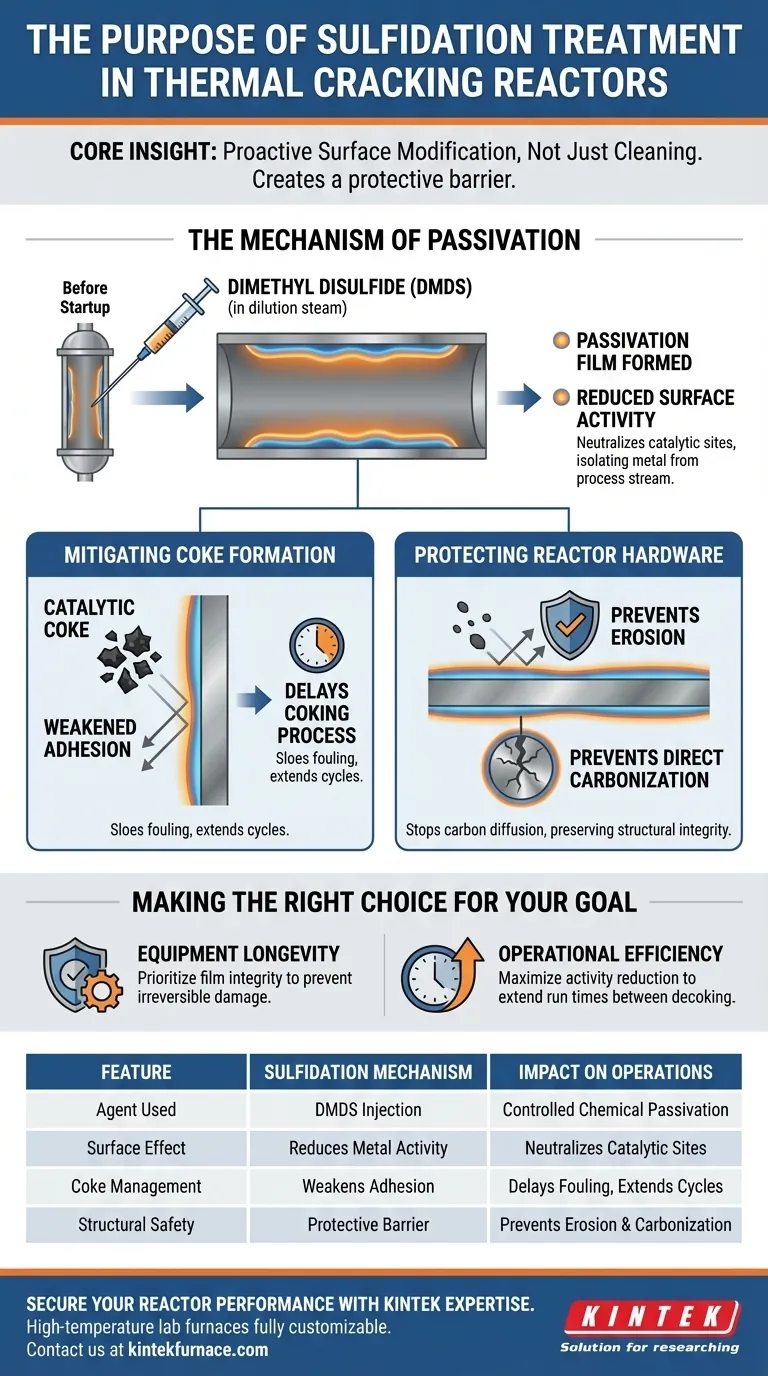
The primary purpose of sulfidation treatment is to establish a protective barrier on the inner walls of a reactor before operations begin. By adding dimethyl disulfide (DMDS) to the dilution steam, this process creates a passivation film that significantly reduces the reactivity of the metal surfaces and inhibits the attachment of coke.
Core Insight: Sulfidation is not just a cleaning step; it is a proactive surface modification. By chemically passivating the reactor walls, you fundamentally alter the metal's surface properties to delay fouling and prevent structural damage from carbonization.

The Mechanism of Passivation
The Role of Dimethyl Disulfide (DMDS)
The process relies on the strategic introduction of dimethyl disulfide (DMDS). This agent is injected into the dilution steam specifically during the pre-startup phase.
The timing is critical. It must occur before the reactor reaches full operational status to ensure the film forms correctly on the clean metal.
Reducing Surface Activity
The immediate chemical result of this treatment is the formation of a passivation film on the tube reactor's inner walls.
Bare metal surfaces in thermal cracking reactors are chemically active. This film neutralizes that activity, effectively isolating the metal from the process stream.
Mitigating Coke Formation
Weakening Adhesion
One of the primary challenges in thermal cracking is the tendency of catalytic coke to adhere to reactor walls.
The sulfidation layer directly addresses this by weakening the adhesion tendency of the coke. This makes it difficult for initial coke deposits to anchor themselves to the tube surface.
Delaying the Coking Process
By reducing metal activity and inhibiting adhesion, the treatment delays the coking process.
It acts as a retardant, slowing the rate at which fouling occurs. This is essential for maintaining heat transfer efficiency during the early stages of the run.
Understanding the Scope of Protection
Protection Against Erosion
Beyond preventing buildup, the film offers physical protection for the reactor hardware.
It shields the reactor substrate from erosion, which can occur due to high-velocity flow and particulate matter within the reactor.
Preventing Direct Carbonization
The film acts as a barrier against direct carbonization of the metal substrate.
Without this layer, carbon could diffuse into the metal, compromising the structural integrity of the reactor walls over time. However, it is important to recognize that this layer delays rather than permanently stops coking; it is a temporary, albeit critical, measure.
Making the Right Choice for Your Goal
To maximize the benefits of sulfidation treatment, consider your specific operational objectives:
- If your primary focus is Equipment Longevity: Prioritize the integrity of the passivation film to protect the reactor substrate from irreversible carbonization and erosion.
- If your primary focus is Operational Efficiency: Use the treatment to maximally reduce metal surface activity, which delays coking and extends the time between required decoking cycles.
A well-executed sulfidation phase is the most effective way to secure both the lifespan of your reactor and the efficiency of your initial run.
Summary Table:
| Feature | Sulfidation Mechanism | Impact on Reactor Operations |
|---|---|---|
| Agent Used | Dimethyl Disulfide (DMDS) | Controlled chemical passivation of metal |
| Surface Effect | Reduces Metal Activity | Neutralizes catalytic sites that trigger coking |
| Coke Management | Weakens Adhesion | Delays fouling and extends production cycles |
| Structural Safety | Protective Barrier | Prevents erosion and direct metal carbonization |
Secure Your Reactor Performance with KINTEK Expertise
Maximize your operational efficiency and protect your equipment from the very first run. KINTEK provides industry-leading thermal solutions backed by expert R&D and manufacturing. Whether you require Muffle, Tube, Rotary, Vacuum, or CVD systems, our high-temperature lab furnaces are fully customizable to meet your unique processing needs.
Don't let coke adhesion and carbonization compromise your results. Contact us today to discover how our specialized equipment and technical insights can optimize your thermal processes!
Visual Guide
References
- P. Nanthagopal R. Sachithananthan. Analytical Review on Impact of Catalytic Coke Formation on Reactor Surfaces During the Thermal Cracking Process. DOI: 10.5281/zenodo.17985551
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What role does a high-temperature furnace play in APTO for Vanadium to VO2? Precision Phase Transformation Explained
- Why is the calcination step essential for Copper Ferrite? Unlock High Purity & Superior Crystallinity
- What is the role of a dedicated bias power supply in low-pressure plasma nitriding? Master Ion Acceleration Control
- Why is a vacuum heating pretreatment system essential for zeolite characterization? Ensure Precise Pore Structure Data
- What is the function of the 1500 °C environment in wood carbonization? Unlock High-Performance Functional Carbon
- What are the advantages of using KOH as a chemical activator? Enhance Biomass Carbon Surface Area and Porosity
- What core processing conditions does a laboratory high-temperature oven provide? Optimize Geopolymer Curing Results
- What are some examples of medium-temperature industrial heating processes? Optimize Material Properties Efficiently















