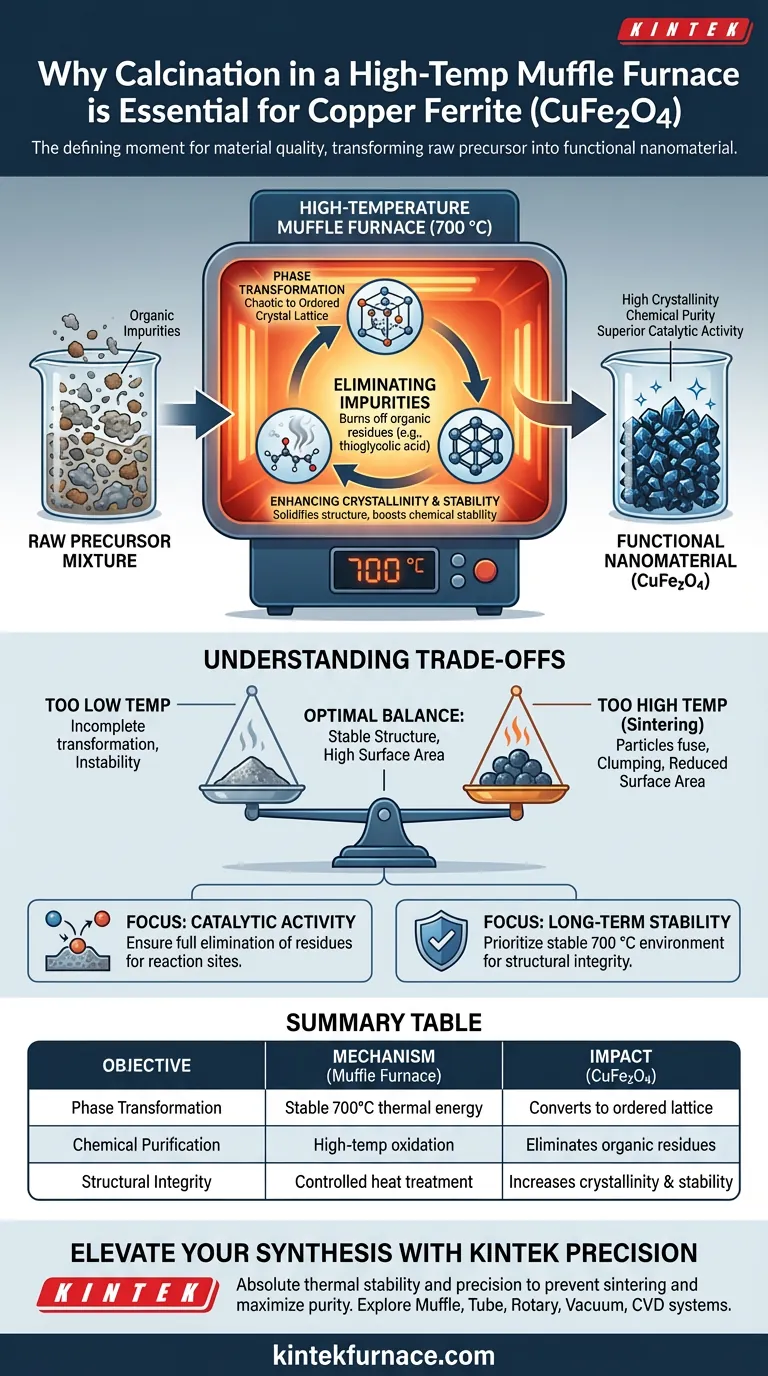
The calcination step is the defining moment for material quality. It transforms the raw precursor mixture into a functional nanomaterial by exposing it to a stable 700 °C environment. This intense heat drives the complete phase transformation of Copper Ferrite (CuFe2O4) while eliminating volatile organic impurities.
Core Takeaway A high-temperature muffle furnace is essential because it provides the thermal energy required to finalize the crystal structure of Copper Ferrite. It ensures the material achieves the high crystallinity and chemical purity necessary for superior catalytic activity and long-term stability.
The Mechanics of Transformation
Driving Complete Phase Transformation
The primary function of the muffle furnace in this context is to provide a stable thermal environment at 700 °C. At this specific temperature, the precursor materials undergo a definitive chemical change.
The heat forces the atoms to rearrange from a chaotic or precursor state into the ordered crystal lattice of Copper Ferrite. Without this stable high-temperature phase, the material would remain an incomplete mixture rather than a unified compound.
Eliminating Organic Impurities
During the synthesis of precursors, organic residues often remain trapped within the material. In the case of Copper Ferrite preparation, residues such as thioglycolic acid may be present.
Calcination acts as a purification step. The high-temperature oxidation environment effectively burns off these organic contaminants. This ensures the final product is chemically pure and free of ligands that could interfere with its performance.
Enhancing Crystallinity and Stability
The application of heat does more than just purify; it solidifies the structural integrity of the material. The 700 °C treatment significantly enhances the crystallinity of the nanomaterials.
Higher crystallinity directly correlates with superior chemical stability. Furthermore, this structural perfection is critical for unlocking the material's potential catalytic activity, making it effective for its intended industrial applications.
Understanding the Trade-offs
Balancing Crystallinity with Surface Area
While high temperatures are necessary for forming the correct phase, there is a delicate balance to maintain. The goal is to achieve a stable crystal structure without applying excessive heat that causes the particles to fuse.
If the temperature is too low, the phase transformation remains incomplete, leading to instability. Conversely, uncontrolled high temperatures can lead to "sintering," where nanoparticles merge into larger clumps, potentially reducing the active surface area available for catalysis.
Making the Right Choice for Your Goal
The muffle furnace is a tool for precision, not just heating. Depending on your specific requirements for the Copper Ferrite, you should adjust your focus as follows:
- If your primary focus is Catalytic Activity: Ensure the calcination fully eliminates thioglycolic acid residues, as surface purity is vital for reaction sites.
- If your primary focus is Long-Term Stability: Prioritize maintaining a stable 700 °C environment to maximize crystallinity, which protects the material from chemical degradation.
Mastering the calcination step is the difference between a volatile precursor mixture and a high-performance industrial catalyst.
Summary Table:
| Process Objective | Mechanism in Muffle Furnace | Impact on Copper Ferrite (CuFe2O4) |
|---|---|---|
| Phase Transformation | Stable 700°C thermal energy | Converts precursors into ordered crystal lattice |
| Chemical Purification | High-temp oxidation | Eliminates organic residues like thioglycolic acid |
| Structural Integrity | Controlled heat treatment | Increases crystallinity and long-term chemical stability |
| Performance Optimization | Precision temp control | Balances catalytic surface area with particle size |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect phase transformation for Copper Ferrite requires more than just heat—it requires the absolute thermal stability and precision of a KINTEK high-temperature muffle furnace. Backed by expert R&D and world-class manufacturing, our systems are engineered to ensure uniform heat distribution, preventing sintering while maximizing crystallinity and purity.
Whether you need Muffle, Tube, Rotary, Vacuum, or CVD systems, KINTEK provides fully customizable lab solutions tailored to your unique research needs. Don't compromise on your catalytic performance. Contact our technical specialists today to find the ideal furnace for your lab and experience the KINTEK difference in high-performance material preparation.
Visual Guide
References
- Soumya Mishra, Prangya Ranjan Rout. Construction of a novel ternary synergistic CuFe <sub>2</sub> O <sub>4</sub> –SnO <sub>2</sub> -rGO heterojunction for efficient removal of cyanide from contaminated water. DOI: 10.1039/d4ra02217c
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is the primary design purpose of industrial electric furnaces for SA-178 Gr A steel? Ensure Pipe End Reliability
- Why is a precision temperature-controlled curing oven required for PIP? Ensure Integrity in Material Cross-Linking
- What is the role of an electric blast drying oven in the pretreatment of SBD? Optimize Your Biomass Research
- What is the purpose of high-purity argon in heat treating Al-Cu-Mn-Zr-V alloys? Protect Your Material Integrity
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure
- Why use a hydraulic press for szaibelyite briquetting? Boost Vacuum Reduction Efficiency & Throughput
- What role does a constant temperature water bath play in simulated hot-rolling oxidation? Master Precision Humidity
- Why is a glove box necessary for aluminum foil pre-lithiation? Ensure Purity in Anode Development



















