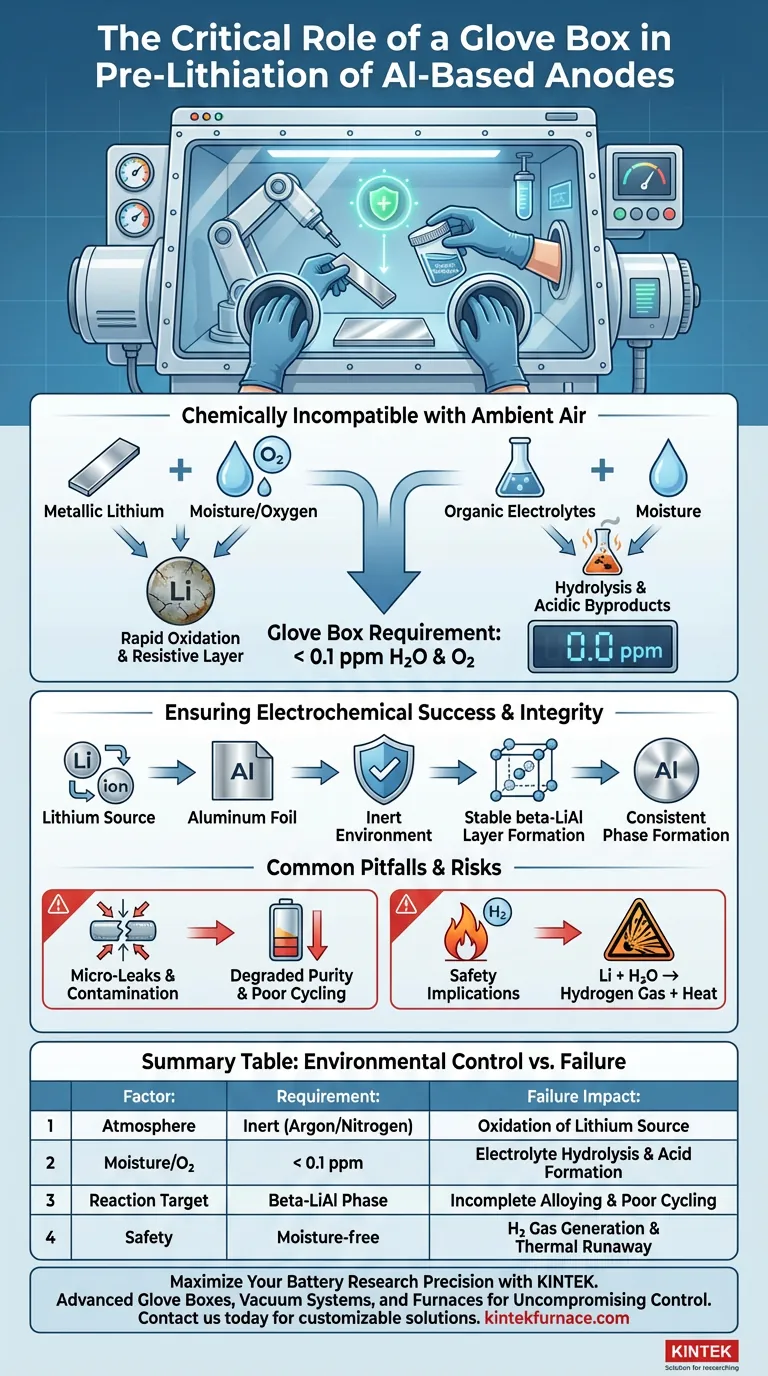
A controlled atmosphere is mandatory for the pre-lithiation of aluminum-based foil anodes because the materials involved are chemically incompatible with ambient air. The process utilizes metallic lithium strips and organic electrolytes, both of which react aggressively with moisture and oxygen. To prevent immediate chemical degradation, a glove box is required to maintain water and oxygen levels strictly below 0.1 ppm.
The integrity of the pre-lithiation process relies on preventing side reactions. Without an inert environment, atmospheric contaminants destroy the lithium source and degrade the electrolyte, making the formation of the necessary beta-LiAl alloy impossible.

The Chemistry of Contamination
The Reactivity of Metallic Lithium
The primary driver for using a glove box is the extreme instability of lithium metal.
When exposed to standard atmosphere, metallic lithium undergoes rapid oxidation. This creates a resistive oxide layer that impedes the transfer of ions, rendering the lithium strip ineffective for the pre-lithiation process.
Electrolyte Stability and Hydrolysis
Organic electrolytes are equally sensitive to the environment.
Moisture in the air triggers hydrolysis, a chemical breakdown of the electrolyte salts and solvents. This not only alters the electrochemical properties of the solution but can also generate acidic byproducts that corrode the aluminum foil.
The 0.1 ppm Standard
Precision is critical in this environment.
The glove box must maintain oxygen and moisture levels below 0.1 parts per million (ppm). This threshold is the industry standard for ensuring that the environment is sufficiently "inert" to stop these rapid degradation reactions.
Ensuring Electrochemical Success
Enabling the Alloying Reaction
The goal of pre-lithiation is to drive a specific electrochemical alloying reaction.
Lithium ions must travel from the source to the aluminum foil to form a stable beta-LiAl layer. Contaminants act as a barrier to this ion flow, causing the process to fail or result in uneven lithiation.
Consistent Phase Formation
A pristine environment ensures the reaction proceeds thermodynamically as intended.
By eliminating side reactions with water or oxygen, the system allows the aluminum to fully accept the lithium. This results in the formation of the correct crystallographic phase (beta-LiAl) required for high-performance anodes.
Common Pitfalls and Risks
The Consequence of Micro-Leaks
Even a microscopic breach in the glove box seal can compromise the batch.
If levels rise above the 0.1 ppm threshold, you may not see immediate failure, but the purity of the beta-LiAl layer will degrade. This often manifests later as poor cycling performance in the final battery cell.
Safety Implications
Beyond process failure, moisture control is a safety imperative.
Lithium metal reacts with water to produce hydrogen gas and heat. In a confined space, maintaining an inert atmosphere is the primary defense against potential thermal runaway or fire hazards.
Optimizing Your Process Environment
If your primary focus is Research Integrity:
- Ensure your glove box sensors are calibrated frequently to guarantee the < 0.1 ppm threshold is actual, not just theoretical.
If your primary focus is Production Yield:
- Implement strict protocols for material transfer to prevent trace moisture introduction during the loading of lithium strips and electrolytes.
The glove box is not merely a storage vessel; it is an active participant in the chemical engineering required to synthesize stable aluminum anodes.
Summary Table:
| Factor | Environmental Requirement | Impact of Failure |
|---|---|---|
| Atmosphere | Inert (Argon/Nitrogen) | Oxidation of metallic lithium source |
| Moisture/O2 | < 0.1 ppm | Electrolyte hydrolysis and acid formation |
| Reaction Target | Beta-LiAl Phase Formation | Incomplete alloying and poor cycling |
| Safety | Moisture-free | Hydrogen gas generation & thermal runaway risk |
Maximize Your Battery Research Precision with KINTEK
High-performance aluminum-based anodes require uncompromising environmental control. At KINTEK, we understand that maintaining sub-0.1 ppm levels is critical for the success of your pre-lithiation process. Backed by expert R&D and manufacturing, KINTEK offers advanced Glove Boxes, Vacuum systems, and customizable lab high-temp furnaces designed to protect your sensitive materials from degradation.
Whether you are scaling up production or conducting fundamental material science, our customizable solutions ensure the integrity of your beta-LiAl alloy formation. Contact us today to discuss how our specialized equipment can enhance your lab’s efficiency and safety.
Visual Guide
References
- Xiaoyang Guo, Steven T. Boles. Holistic Processing of Sawdust to Enable Sustainable Hybrid Li-Ion Capacitors. DOI: 10.1007/s11837-024-06542-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the importance of controlling gas flow rates during purging? Prevent Thermal Stress and Equipment Failure
- Why is a vibratory mill used for ultra-fine grinding when preparing magnesite samples for zeta potential tests?
- How does a lab high-temp furnace ensure the integrity of quasicrystal-reinforced aluminum composites? Expert Guide
- Why is high-precision temperature control at 800 °C critical for BCMoMn catalyst heterostructures?
- What is the primary function of a high-precision program-controlled furnace? Mastering T6 Heat Treatment of Al-Cu 224
- How is SEM utilized to evaluate manganese phosphate catalysts after calcination? Verify Nanosheet Integrity.
- What is the purpose of annealing the sapphire substrate at 980 °C with Cr? Achieve Unidirectional Cr2S3 Growth
- How does a vacuum drying oven contribute to biodiesel moisture control? Ensure Fuel Quality & Stability





