High-precision temperature control at exactly 800 °C is the thermodynamic prerequisite for successfully synthesizing high-performance BCMoMn catalyst heterostructures. This specific thermal environment ensures the uniform energy distribution necessary to drive the full conversion of metal intermediates into active clusters while establishing critical electronic linkups.
Precision at 800 °C is the singular condition that allows for the complete formation of Mo2C and Mn7C3 clusters and their electronic coupling with MnN4 sites, striking a balance that prevents both structural underdevelopment and thermal degradation.
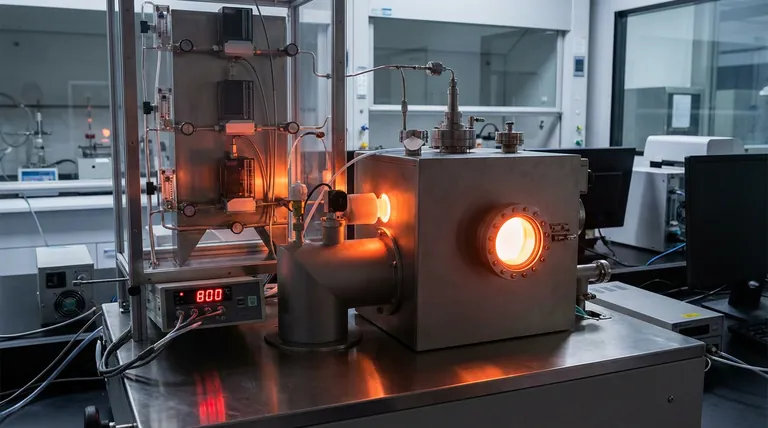
The Mechanics of Formation at 800 °C
To understand why this specific temperature is non-negotiable, one must look at the microscopic changes occurring within the catalyst material.
Uniform Energy Distribution
At 800 °C, the thermal equipment provides a consistent and uniform energy landscape.
This uniformity is required to activate the metal intermediates simultaneously across the material structure.
Without this precise energy input, the chemical transformation remains patchy and inconsistent.
Complete Conversion of Intermediates
The primary goal of this thermal stage is the conversion of precursors into specific active clusters.
Specifically, the 800 °C environment drives the full transformation of metal intermediates into Mo2C (Molybdenum Carbide) and Mn7C3 (Manganese Carbide) clusters.
These clusters are the fundamental building blocks of the catalyst's reactivity.
Inducing Electronic Coupling
Beyond simple formation, the components must interact electronically to function as a heterostructure.
The 800 °C threshold induces strong electronic coupling between the newly formed clusters and MnN4 single-atom sites.
This electronic synergy is what ultimately defines the high performance of the BCMoMn catalyst.
Understanding the Trade-offs (Consequences of Deviation)
In catalyst synthesis, 800 °C is not merely a suggestion; it is a critical tipping point. Deviating even by 100 °C in either direction compromises the material's integrity.
The Cost of Lower Temperatures (700 °C)
Operating at 700 °C fails to provide the activation energy required for full conversion.
This results in underdeveloped active sites, as the metal intermediates do not completely transform into the necessary carbide clusters.
The resulting material lacks the density of active sites required for effective catalysis.
The Risk of Higher Temperatures (900 °C)
Exceeding the threshold and operating at 900 °C introduces destructive thermal energy.
This leads to the over-consumption of the material or the coarsening of the clusters.
Coarsening reduces the surface area and destroys the delicate nanostructure, rendering the catalyst less effective despite the high energy input.
Making the Right Choice for Your Synthesis
When designing your synthesis protocol or troubleshooting catalyst performance, strict thermal management is your primary variable.
- If your primary focus is maximizing catalytic activity: Calibrate your equipment to maintain exactly 800 °C to ensure strong coupling between MnN4 sites and fully formed carbide clusters.
- If your primary focus is troubleshooting low performance: Analyze the material structure; underdeveloped sites suggest thermal gradients below 800 °C, while coarse grains suggest overshooting toward 900 °C.
Ultimately, the formation of a functional BCMoMn heterostructure relies entirely on hitting this precise thermal window to balance conversion with structural preservation.
Summary Table:
| Temperature (°C) | Synthesis Outcome | Effect on Heterostructure |
|---|---|---|
| 700 °C | Underdeveloped | Incomplete conversion of metal intermediates into active clusters |
| 800 °C | Optimal Formation | Full Mo2C/Mn7C3 conversion and strong electronic coupling with MnN4 |
| 900 °C | Thermal Degradation | Material over-consumption and cluster coarsening/nanostructure loss |
Unlock High-Performance Catalyst Synthesis with KINTEK
Precision is the difference between a high-performance heterostructure and a failed experiment. KINTEK provides the specialized thermal equipment needed to maintain the exact 800 °C environment required for BCMoMn development.
Our Value to Your Laboratory:
- Expert R&D & Manufacturing: Our systems are engineered for the extreme temperature uniformity essential for catalytic conversion.
- Versatile Solutions: Choose from our Muffle, Tube, Rotary, Vacuum, and CVD systems to suit your specific gas-flow and atmospheric needs.
- Tailored Customization: We customize every furnace to meet your unique research parameters, preventing the structural underdevelopment or thermal degradation of your materials.
Ensure your catalyst precursors achieve full electronic coupling. Contact KINTEK today to discuss your furnace requirements!
References
- Chengyu Zhang, Zhisheng Yu. Electronic configuration regulation of single-atomic Mn sites mediated by Mo/Mn clusters for an efficient hydrogen evolution reaction. DOI: 10.1039/d3sc06053e
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What are the uses of furnace in laboratory? The Essential Tool for Material Transformation
- What is the temperature of a sintering furnace? From 1100°C to 2200°C+ for Your Material
- What are the advantages of a benchtop industrial oven in terms of usability? Enhance Lab Efficiency with Compact Design
- Why is a vacuum drying oven required for precursor mixtures? Achieve Stable, High-Quality Powder Processing
- Why is high-temperature hydrogen reduction used for HI decomposition catalysts? Boost Efficiency and Surface Purity
- What is the function of a pure graphite sheet within a microwave hybrid heating setup? Ensure Pure Ni-BN Cladding
- What are the core advantages of using a microwave hydrothermal synthesis system? Rapid & Uniform CNS Production
- How does metallic magnesium facilitate deep purification of molten chloride salts at 800 °C? Achieve Ultra-High Purity



















