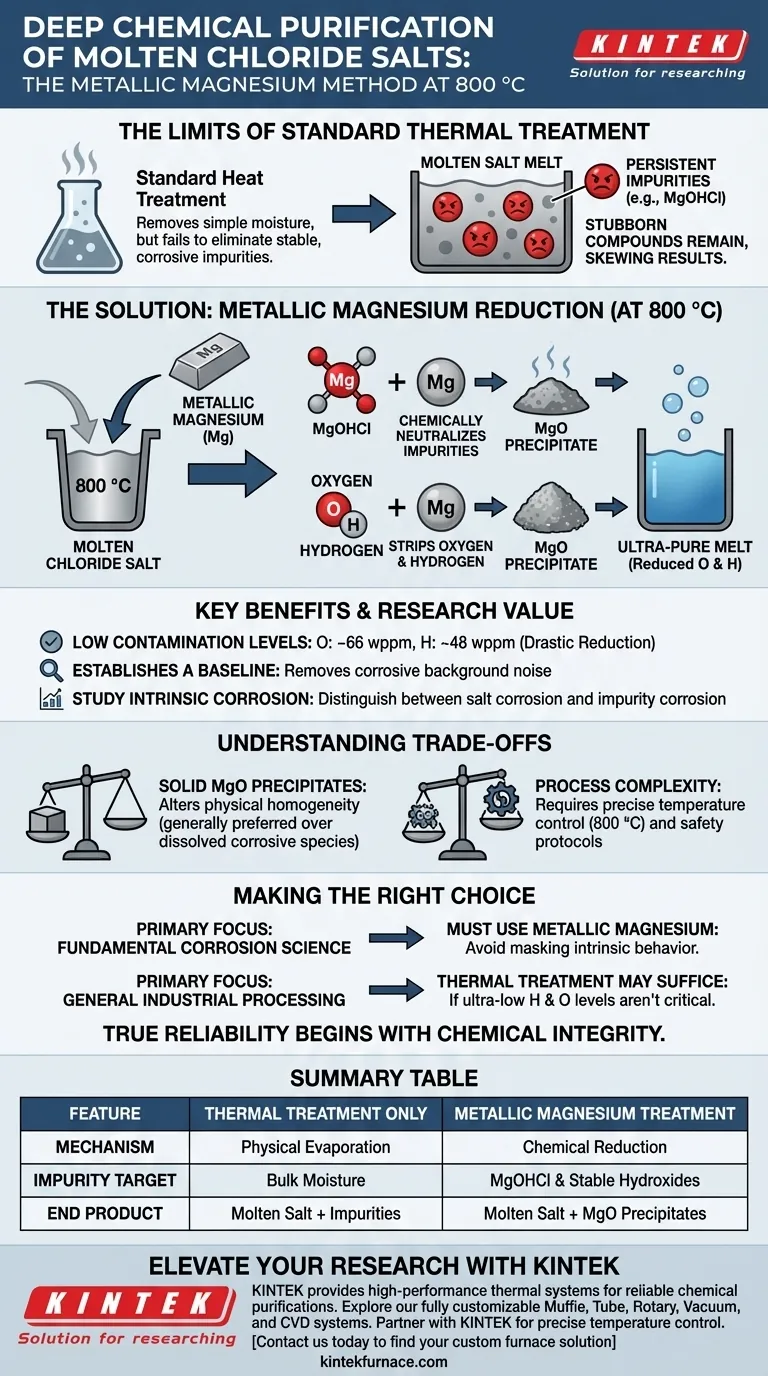
The addition of metallic magnesium functions as a potent reducing agent that chemically neutralizes impurities surviving standard heat treatments. When introduced to molten chloride salts at 800 °C, magnesium reacts with stubborn compounds like magnesium hydroxychloride (MgOHCl), converting them into insoluble magnesium oxide (MgO) precipitates and effectively stripping oxygen and hydrogen from the melt.
While thermal treatment removes bulk moisture, it often fails to eliminate stable corrosive species. Metallic magnesium solves this by chemically reducing these impurities, achieving the ultra-high purity levels necessary for isolating intrinsic material corrosion behavior.

The Limits of Thermal Treatment
The Persistence of Hydroxychlorides
Standard heat treatments are effective at driving off simple moisture from salts. However, they often fail to remove chemically bound impurities.
The Specific Challenge of MgOHCl
Specifically, compounds like magnesium hydroxychloride (MgOHCl) remain stable even at high temperatures. These impurities are highly corrosive and can significantly skew experimental results if left in the melt.
The Chemical Mechanism of Purification
Magnesium as a Reducing Agent
At 800 °C, metallic magnesium is highly reactive. It targets the oxygen and hydrogen bonds within the remaining impurities.
Formation of MgO Precipitates
The magnesium reacts with dissolved hydroxides to form magnesium oxide (MgO). Unlike the dissolved impurities, MgO forms a solid precipitate that separates from the liquid salt chemistry.
Deep Reduction of Contaminants
This reaction drives a drastic reduction in contamination levels. The process has been shown to lower oxygen concentrations to 66 wppm and hydrogen concentrations to 48 wppm.
The Strategic Value for Research
Establishing a Baseline
The primary goal of this deep purification is to create a "neutral" environment. By removing corrosive background noise, researchers can observe the true interaction between the salt and the container materials.
Studying Intrinsic Corrosion
Without deep purification, it is impossible to distinguish between corrosion caused by the salt itself and corrosion caused by impurities. This method isolates the variable, allowing for accurate studies of intrinsic material degradation.
Understanding the Trade-offs
Managing Precipitates
While the conversion to MgO removes dissolved oxygen, it introduces solid particulates into the melt. These precipitates are generally preferred over dissolved corrosive species, but they technically alter the physical homogeneity of the fluid.
Process Complexity
Operating at 800 °C with reactive metallic magnesium requires precise thermal control and safety protocols. This adds a layer of operational complexity compared to simple drying or baking procedures.
Making the Right Choice for Your Goal
To determine if this purification step is necessary for your application, consider the following:
- If your primary focus is fundamental corrosion science: You must use metallic magnesium to remove MgOHCl, as dissolved impurities will mask the intrinsic behavior of the material you are testing.
- If your primary focus is general industrial processing: Simple thermal treatment may suffice if ultra-low hydrogen (48 wppm) and oxygen levels are not critical to your process efficiency.
True reliability in molten salt data begins with the chemical integrity of the melt itself.
Summary Table:
| Feature | Thermal Treatment Only | Metallic Magnesium Treatment |
|---|---|---|
| Mechanism | Physical evaporation | Chemical reduction |
| Impurity Target | Bulk moisture | MgOHCl and stable hydroxides |
| Oxygen Level | High (Residual) | ~66 wppm |
| Hydrogen Level | High (Residual) | ~48 wppm |
| End Product | Molten salt + impurities | Molten salt + MgO precipitates |
| Best Use Case | General industrial processing | Fundamental corrosion research |
Elevate Your Research with Ultra-Pure Salt Environments
Precise corrosion data depends on the integrity of your melt. KINTEK provides the high-performance thermal systems necessary to execute complex chemical purifications with absolute reliability. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temperature furnaces—all fully customizable to meet your unique experimental needs.
Don't let corrosive impurities mask your results. Partner with KINTEK to achieve the precise temperature control required for deep chemical reduction. Contact us today to find your custom furnace solution!
Visual Guide
References
- Mingyang Zhang, Jinsuo Zhang. Corrosion kinetics of pure metals (Fe, Cr, Ni) and alloys (A709, SS316) in thermal and chemical purified molten chloride salt. DOI: 10.1039/d5ra00451a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Which type of furnace is better for specific applications? Choose the Right Furnace for Your Production Needs
- Why is the initial concentration of siloxane systems performed in a vacuum oven? Achieve Defect-Free Material Curing
- What role does a high-temperature furnace play in the sealing process? Precision Thermal Control for Fiber Sealing
- What role does quartz sand filler play in a crystal growth furnace? Enhance Thermal Symmetry and Yield
- How does Oxygen-Enhanced Combustion (OEC) improve furnace thermal efficiency? Boost Energy Savings and Heat Recovery
- Why is the intervention of precision heat treatment equipment essential for AlSi10Mg parts? Enhance LPBF Integrity
- What happens during the sintering process? Transform Powder into Dense, High-Strength Components
- What is the function of solution and aging heat treatment furnaces? Optimize 17-4 PH Stainless Steel Properties



















