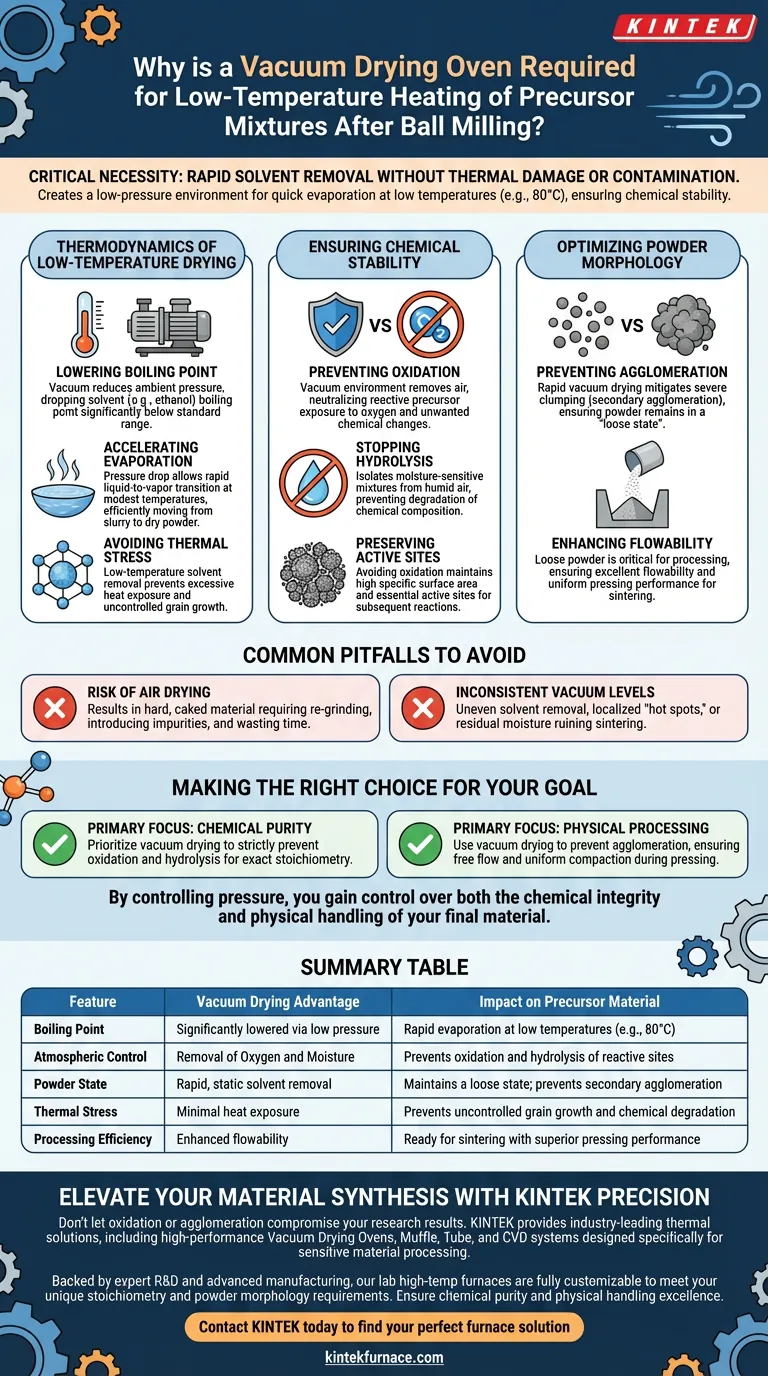
The critical necessity of a vacuum drying oven lies in its ability to rapidly remove solvents from post-milling slurries without subjecting the material to thermal damage or atmospheric contamination. By creating a low-pressure environment, the oven allows solvents like ethanol to evaporate quickly at temperatures as low as 80°C, ensuring the precursor remains chemically stable.
The vacuum environment fundamentally alters the drying dynamics by lowering the solvent's boiling point. This allows for rapid drying that prevents oxidation and agglomeration, yielding a loose, high-quality powder ready for sintering.

The Thermodynamics of Low-Temperature Drying
Lowering the Boiling Point
The primary mechanism at work is the reduction of ambient pressure. In a vacuum, the boiling point of solvents like ethanol drops significantly below their standard range.
Accelerating Evaporation
This pressure drop allows the liquid phase to evaporate rapidly, even at modest temperatures (e.g., 80°C). This speed is essential for moving from a slurry state to a dry powder efficiently.
Avoiding Thermal Stress
Because the solvent boils off at a lower temperature, the precursor material is not exposed to excessive heat. This prevents uncontrolled grain growth that often occurs during high-temperature drying processes.
Ensuring Chemical Stability
Preventing Oxidation
Standard air drying exposes reactive precursors to oxygen, leading to unwanted chemical changes. A vacuum environment removes air from the chamber, effectively neutralizing the risk of oxidation.
Stopping Hydrolysis
Many precursor mixtures are sensitive to moisture in the air. By drying in a vacuum, you isolate the material from humid air, preventing hydrolysis reactions that would degrade the chemical composition of the mixture.
Preserving Active Sites
For advanced materials like nanosheets, avoiding oxidation preserves the material's high specific surface area. This maintains the essential active sites required for subsequent compositing or reactions.
Optimizing Powder Morphology
Preventing Agglomeration
Drying in a static, non-vacuum environment often leads to severe clumping, or secondary agglomeration. Rapid vacuum drying mitigates this, ensuring the powder remains in a "loose state."
Enhancing Flowability
A loose, non-agglomerated powder is critical for the next stage of processing. It ensures excellent flowability and pressing performance, allowing the material to be easily transferred to sintering crucibles or molded into shapes.
Common Pitfalls to Avoid
The Risk of Air Drying
Attempting to bypass the vacuum stage and using a standard air oven is a common error. This typically results in a hard, caked material that requires re-grinding, introducing impurities and wasting time.
Inconsistent Vacuum Levels
If the vacuum pressure is not maintained consistently, solvent removal becomes uneven. This can lead to localized "hot spots" in the powder or residual moisture that ruins the sintering process later.
Making the Right Choice for Your Goal
To maximize the quality of your precursor powder, align your drying strategy with your specific material requirements:
- If your primary focus is Chemical Purity: Prioritize vacuum drying to strictly prevent oxidation and hydrolysis, ensuring the stoichiometry remains exact.
- If your primary focus is Physical Processing: Use vacuum drying to prevent agglomeration, ensuring the powder flows freely and compacts uniformly during pressing.
By controlling pressure, you gain control over both the chemical integrity and physical handling of your final material.
Summary Table:
| Feature | Vacuum Drying Advantage | Impact on Precursor Material |
|---|---|---|
| Boiling Point | Significantly lowered via low pressure | Rapid evaporation at low temperatures (e.g., 80°C) |
| Atmospheric Control | Removal of Oxygen and Moisture | Prevents oxidation and hydrolysis of reactive sites |
| Powder State | Rapid, static solvent removal | Maintains a loose state; prevents secondary agglomeration |
| Thermal Stress | Minimal heat exposure | Prevents uncontrolled grain growth and chemical degradation |
| Processing Efficiency | Enhanced flowability | Ready for sintering with superior pressing performance |
Elevate Your Material Synthesis with KINTEK Precision
Don't let oxidation or agglomeration compromise your research results. KINTEK provides industry-leading thermal solutions, including high-performance Vacuum Drying Ovens, Muffle, Tube, and CVD systems designed specifically for sensitive material processing.
Backed by expert R&D and advanced manufacturing, our lab high-temp furnaces are fully customizable to meet your unique stoichiometry and powder morphology requirements. Ensure chemical purity and physical handling excellence—Contact KINTEK today to find your perfect furnace solution.
Visual Guide
References
- Jiadong Chen, Wenhao Sun. Navigating phase diagram complexity to guide robotic inorganic materials synthesis. DOI: 10.1038/s44160-024-00502-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why are continuous furnaces ideal for high-volume manufacturing? Boost Throughput and Consistency
- What are some common applications of laboratory furnaces? Unlock Precision in Material Transformation
- Why is a steam generator and programmable furnace needed for emission aging? Replicate Real Hydrothermal Environments
- What are the functions of a rotary evaporator and a vacuum drying oven in LTO sol-gel? Optimize Your Synthesis Process
- Why is it necessary to use a vacuum drying oven for porous graphene cathodes? Ensure Peak Battery Performance
- What role does an industrial electric furnace play in PAI? Master Thermal Preparation for Metal Matrix Composites
- What is the significance of using high-temperature heating equipment to reach 1250°C for alloys? Stress Test Excellence
- Why is the mechanical mixing of precursor powders necessary for ITO thin films? Guide to Precision Growth



















