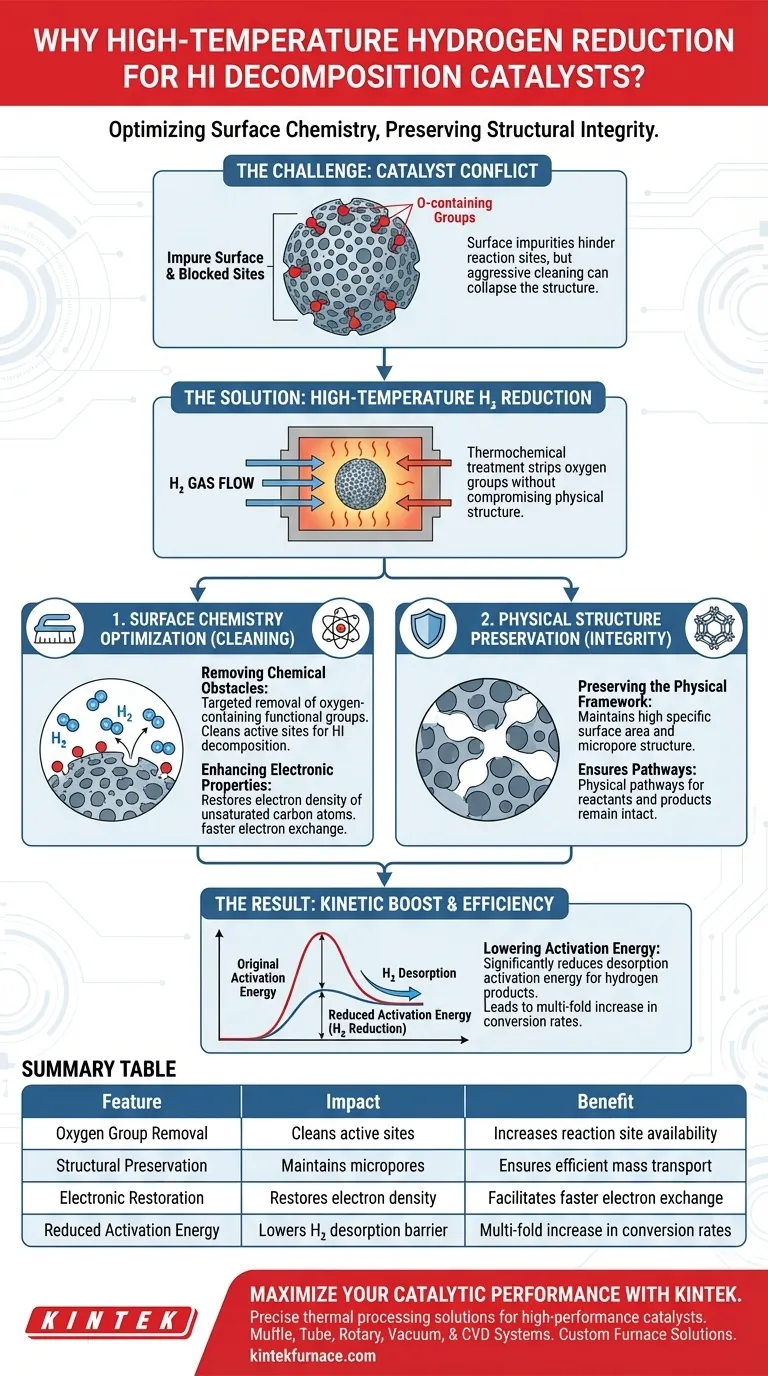
High-temperature hydrogen reduction is the recommended treatment for hydrogen iodide (HI) decomposition catalysts because it fundamentally optimizes the catalyst's surface chemistry without compromising its physical structure. By utilizing thermochemical reactions to strip away oxygen-containing functional groups, this process significantly lowers the energy barrier required for hydrogen desorption, leading to a multi-fold increase in conversion efficiency.
The core value of this treatment lies in its ability to resolve the conflict between surface purity and structural integrity. It removes chemical impurities that hinder reaction sites while preserving the critical micropore structure, directly translating to higher catalytic activity.

The Mechanics of Surface Modification
Removing Chemical Obstacles
The primary function of high-temperature hydrogen reduction is the targeted removal of oxygen-containing functional groups.
Through thermochemical reactions, hydrogen strips these groups from the catalyst surface. This effectively "cleans" the active sites, preparing them for the specific demands of HI decomposition.
Preserving the Physical Framework
A common risk in catalyst modification is the collapse of the material's internal architecture.
However, this specific treatment maintains the material's high specific surface area and its developed micropore structure. This ensures that the physical pathways required for reactants to enter and products to exit remain intact.
Enhancing Electronic and Kinetic Properties
Restoring Electron Density
Beyond physical cleaning, this treatment alters the electronic state of the catalyst material, specifically activated carbon.
It restores the electron density of unsaturated carbon atoms. This electronic restoration is critical for facilitating the exchange of electrons during the catalytic reaction.
Lowering Activation Energy
The efficiency of a catalyst is often bottled-necked by how easily it releases the final product.
This treatment significantly reduces the desorption activation energy of hydrogen products. By lowering this energy barrier, the catalyst can release hydrogen more freely, directly increasing the reaction rate.
Understanding the Trade-offs
The Balance of Structure vs. Chemistry
In many surface modification processes, aggressive chemical treatments often degrade the porous structure of the support material.
The distinct advantage—and necessary trade-off to manage—of this method is achieving deep chemical modification (removal of oxygen groups) while strictly avoiding the degradation of micropores. If the treatment temperature or duration is not precisely controlled to match the material's tolerance, one risks altering the physical properties that are explicitly meant to be preserved.
Making the Right Choice for Your Goal
To maximize the performance of your HI decomposition catalysts, apply this treatment based on your specific optimization targets:
- If your primary focus is Kinetic Efficiency: Utilize this treatment to lower desorption activation energy, allowing for faster product release and higher turnover rates.
- If your primary focus is Structural Integrity: Rely on this method to modify surface chemistry while strictly maintaining the specific surface area and pore volume required for mass transport.
This treatment provides the rare combination of electronic optimization and physical preservation, making it indispensable for high-performance catalysis.
Summary Table:
| Feature | Impact on Catalyst Performance | Benefit to HI Decomposition |
|---|---|---|
| Oxygen Group Removal | Cleans active sites by stripping impurities | Increases reaction site availability |
| Structural Preservation | Maintains micropores and surface area | Ensures efficient mass transport |
| Electronic Restoration | Restores electron density of carbon atoms | Facilitates faster electron exchange |
| Reduced Activation Energy | Lowers hydrogen desorption energy barrier | Multi-fold increase in conversion rates |
Maximize Your Catalytic Performance with KINTEK
Precise thermal processing is the key to unlocking the full potential of your high-performance catalysts. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temperature furnaces—all fully customizable to meet your unique research and production needs.
Whether you are refining HI decomposition processes or developing next-generation materials, our precision heating solutions ensure you achieve the perfect balance between chemical modification and structural integrity.
Ready to elevate your lab's efficiency? Contact us today to find your custom furnace solution!
Visual Guide
References
- Xuhan Li, Liqiang Zhang. Boosting Hydrogen Production from Hydrogen Iodide Decomposition over Activated Carbon by Targeted Removal of Oxygen Functional Groups: Evidence from Experiments and DFT Calculations. DOI: 10.3390/en18164288
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How do thermal systems reveal anti-spalling mechanisms in CDE concrete? Explore Advanced Material Resilience
- Why is a constant temperature drying oven used at 120°C for 16 hours for NiCuCe catalysts? Optimize Site Dispersion
- What role does a laboratory oven play in the drying phase of Co–Mg catalyst precursors? Ensuring Component Uniformity
- What role does an industrial electric furnace play in PAI? Master Thermal Preparation for Metal Matrix Composites
- What are the advantages of PVD equipment for solar absorber films? Achieve Nanometer Precision and Maximum Efficiency
- What are the primary functions of a high-precision dilatometer in hot ductility? Optimize Steel Casting Precision
- What is the importance of using a vacuum drying oven for MoS2/rGO battery electrodes? Maximize Battery Performance
- What is the temperature of a sintering furnace? From 1100°C to 2200°C+ for Your Material



















