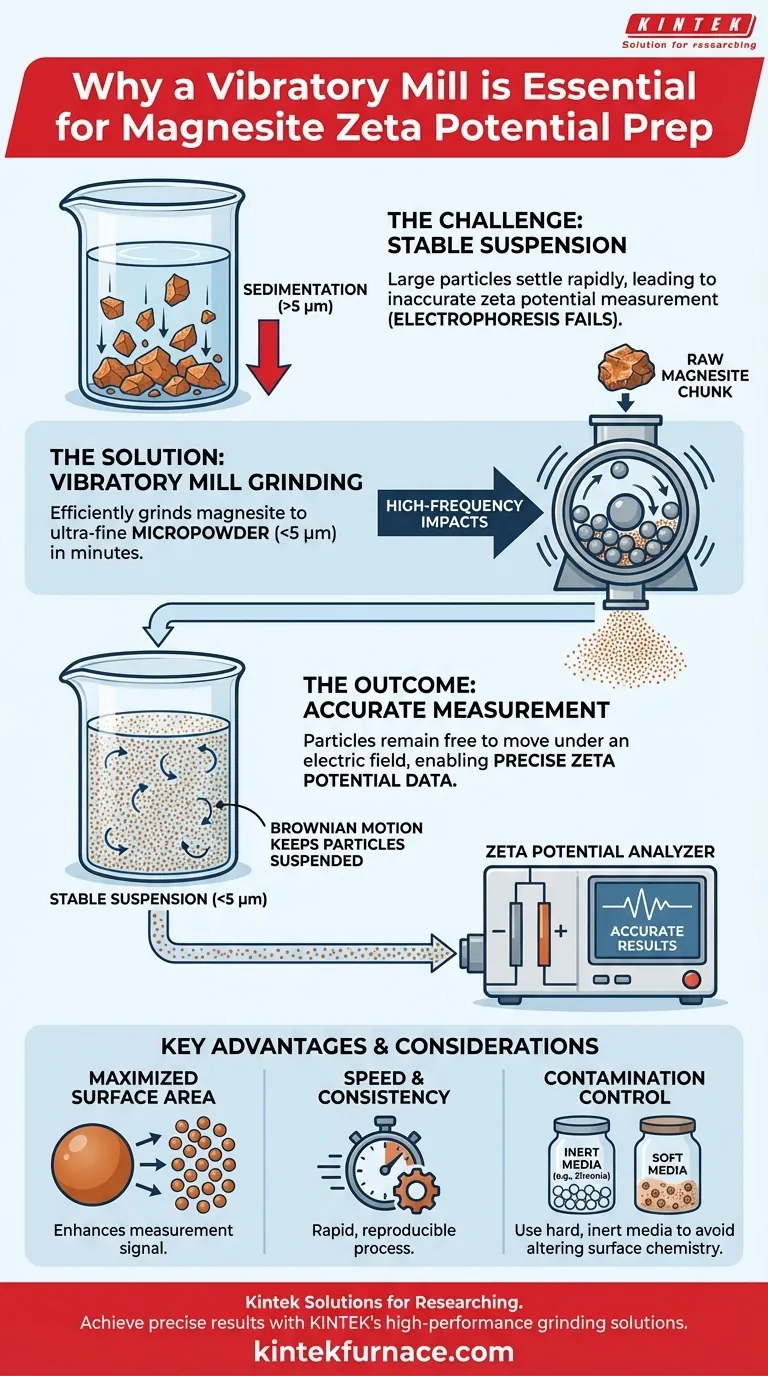
In short, a vibratory mill is used because it is a highly efficient method for rapidly grinding magnesite into an ultra-fine powder, typically smaller than 5 micrometers. This specific particle size is non-negotiable for creating the stable particle suspension required for an accurate zeta potential measurement.
The accuracy of a zeta potential test is fundamentally dependent on the physical characteristics of the sample being measured. The primary role of the vibratory mill is to transform a raw mineral sample into a physically ideal micropowder that will remain suspended in liquid, allowing its surface electrical properties to be precisely analyzed.

The Physics Behind the Preparation
To understand the choice of equipment, we must first understand the demands of the measurement itself. A zeta potential test does not measure a solid chunk of material; it measures the behavior of tiny particles dispersed in a liquid.
The Basis of Measurement: Electrophoresis
Zeta potential is determined by applying an electric field to a liquid suspension and measuring the velocity of the particles within it. This technique is known as electrophoresis.
Accurate measurement is only possible if the particles are free to move and, most importantly, do not settle to the bottom of the container during the test.
Why a Stable Suspension is Critical
If particles are too large or heavy, gravity will quickly pull them out of the liquid, a process called sedimentation.
When particles settle, they are no longer part of the suspension and cannot be measured. This leads to inaccurate and unreliable data, rendering the experiment useless. The goal of sample preparation is to prevent this at all costs.
The Link Between Particle Size and Stability
A vibratory mill's key function is to drastically reduce particle size. By grinding magnesite to below 5 micrometers, the particles become so small and light that the random, constant motion of liquid molecules (Brownian motion) is sufficient to counteract gravity and keep them suspended.
This creates the stable, homogenous suspension that is the absolute prerequisite for any meaningful zeta potential test.
Key Advantages of the Vibratory Mill
While other grinding methods exist, the high-frequency vibratory mill is uniquely suited for this task due to its efficiency and the specific qualities of the powder it produces.
Achieving Ultra-Fine Particle Size
The mill uses high-frequency vibrations to cause grinding media (like balls or cylinders) to collide with the sample material thousands of times per minute. This high-energy impact is extremely effective at breaking down crystalline materials like magnesite to the required micropowder or ultra-fine state.
Maximizing Specific Surface Area
Zeta potential is a measure of the electrical charge at the particle-liquid interface. It is exclusively a surface property.
Grinding a sample into smaller particles dramatically increases its specific surface area (the total surface area per unit of mass). This larger surface area provides a stronger, more representative signal for the measurement equipment, enhancing the accuracy of the results.
Speed and Consistency
Vibratory mills are known for their rapid grinding times. This efficiency is a practical advantage in a laboratory setting, allowing for higher throughput and ensuring the sample's surface properties do not change over long processing times.
Understanding the Potential Pitfalls
While highly effective, using a high-energy milling process requires careful consideration to avoid introducing new sources of error into your experiment.
Risk of Sample Contamination
The grinding media and the mill's chamber can wear down during the aggressive grinding process. This can introduce contaminants into your magnesite sample.
If the contaminating material has a different surface chemistry, it can significantly alter the measured zeta potential. Choosing a hard, inert grinding media (like zirconia or agate) is crucial to minimize this effect.
Impact of Heat Generation
The high energy involved in vibratory milling generates heat. For some sensitive materials, this heat could potentially alter the mineral's surface chemistry or crystal structure, again leading to skewed results.
Making the Right Choice for Your Goal
Proper sample preparation is not just a preliminary step; it is an integral part of the measurement. Your approach should be guided by the ultimate goal of your analysis.
- If your primary focus is accuracy: You must prioritize achieving the target particle size (<5 µm) to guarantee a stable suspension, as this is the foundation of a valid measurement.
- If your primary focus is reproducibility: Implement and document a strict, standardized grinding protocol—including milling time, intensity, and sample mass—to ensure all your samples are physically comparable.
- If your primary focus is chemical purity: Carefully select your grinding media to be as inert and hard as possible to prevent surface contamination from skewing your electrochemical data.
Ultimately, the quality of your sample preparation directly determines the quality and trustworthiness of your final results.
Summary Table:
| Requirement | Role of Vibratory Mill |
|---|---|
| Particle Size (<5µm) | Achieves ultra-fine grinding via high-frequency impacts. |
| Stable Suspension | Prevents sedimentation, enabling accurate electrophoresis. |
| Maximized Surface Area | Enhances signal strength for zeta potential measurement. |
| Contamination Control | Requires inert media (e.g., zirconia) to preserve sample purity. |
Achieve Precise Zeta Potential Results with KINTEK's Grinding Solutions
Your zeta potential analysis is only as reliable as your sample preparation. KINTEK's high-performance vibratory mills are engineered to deliver the ultra-fine, consistent particle size critical for stable suspensions and accurate electrochemical data.
Our expertise ensures:
- Rapid, reproducible grinding to sub-5µm specifications.
- Minimized contamination with customizable, inert grinding media.
- Enhanced measurement accuracy through optimized specific surface area.
Backed by expert R&D and manufacturing, KINTEK offers a full range of laboratory mills and furnaces, all customizable for your unique research needs.
Ready to optimize your magnesite sample preparation? Contact our experts today for a tailored solution.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the key role of a muffle furnace in the pretreatment of boron sludge and szaibelyite? Unlock Higher Process Efficiency
- What metals cannot be heated by induction? Understanding Material Suitability for Efficient Heating
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- What is the role of a muffle furnace in the synthesis of water-soluble Sr3Al2O6? Precision in SAO Production
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production















