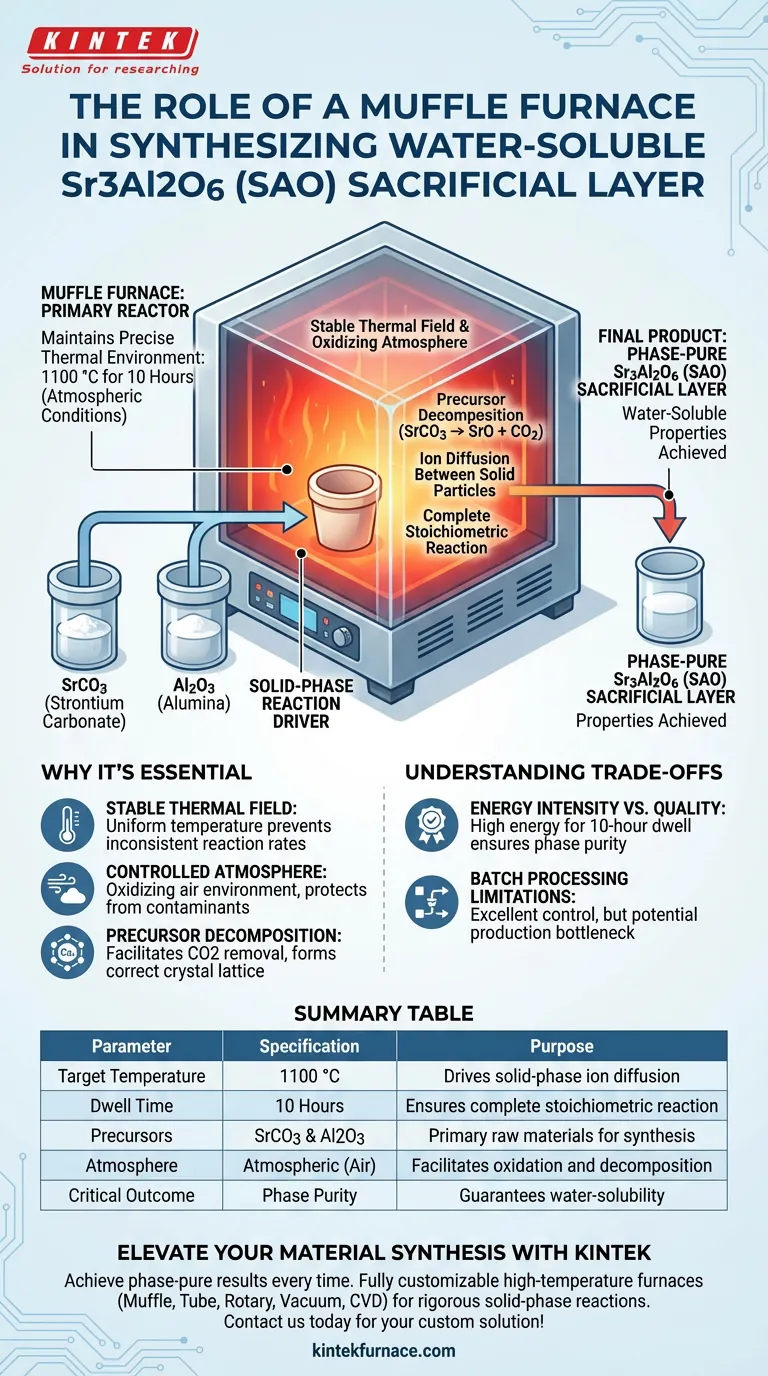
The muffle furnace functions as the primary reactor for the high-temperature solid-phase synthesis of Sr3Al2O6 (SAO). It maintains a precise thermal environment at 1100 °C for 10 hours under atmospheric conditions, driving the chemical reaction between strontium carbonate (SrCO3) and alumina (Al2O3) precursors. This prolonged thermal treatment is mandatory to convert raw powders into a phase-pure, stoichiometric target material suitable for use as a water-soluble sacrificial layer.
Core Takeaway The muffle furnace does not simply heat the material; it provides the stable thermal field and oxidizing atmosphere necessary to ensure a complete solid-phase reaction. Without this specific thermal profile, the precursors would fail to achieve the precise stoichiometric ratio required for the material's water-soluble properties.
The Mechanism of SAO Synthesis
Driving the Solid-Phase Reaction
The synthesis of Sr3Al2O6 is not a simple melting process; it is a solid-phase reaction. The muffle furnace provides the energy required to diffuse ions between the solid particles of Strontium Carbonate and Alumina.
Achieving Stoichiometric Precision
The furnace must hold the material at 1100 °C for an extended period, specifically 10 hours. This dwell time ensures the reaction is complete throughout the entire powder volume, resulting in a target material with a precise stoichiometric ratio.
Ensuring Phase Purity
Incomplete heating results in secondary phases or unreacted precursors. The muffle furnace ensures high phase purity, which is critical for the material's performance as a sacrificial layer. Impurities could alter the solubility rate or leave insoluble residues during the etching process.
Why a Muffle Furnace is Essential
Stable Thermal Field
As seen in similar material processing contexts, muffle furnaces are prized for creating a uniform and stable thermal field. This uniformity prevents temperature gradients that could lead to inconsistent reaction rates within a single batch of powder.
Controlled Atmospheric Environment
The synthesis of SAO requires an atmospheric environment (air). The muffle furnace design allows for this oxidizing atmosphere while protecting the sample from direct contact with heating elements or fuel contaminants.
Precursor Decomposition
Before the final phase formation, the furnace heat facilitates the decomposition of carbonate precursors (SrCO3). This effectively removes carbon dioxide and allows the remaining oxides to form the correct crystal lattice.
Understanding the Trade-offs
Energy Intensity vs. Material Quality
The requirement for a 10-hour dwell time at 1100 °C makes this process energy-intensive. You are trading energy efficiency for the guarantee of high phase purity and complete reaction.
Batch Processing Limitations
Muffle furnaces are typically batch-processing units. While they offer excellent control for high-value materials like SAO, they may act as a bottleneck in high-volume production compared to continuous flow furnaces.
Thermal Lag
Large muffle furnaces may have significant thermal mass. You must account for heating and cooling ramp rates to avoid thermal shock to the ceramic crucibles or the material itself, although this is generally less critical for powders than for sintered parts.
Making the Right Choice for Your Goal
To maximize the effectiveness of your Sr3Al2O6 synthesis, align your furnace operation with your specific project needs:
- If your primary focus is Phase Purity: Adhere strictly to the 1100 °C and 10-hour protocol; shortening this time to save energy risks incomplete reactions and insoluble residues.
- If your primary focus is Process Efficiency: Investigate the maximum packing density of your crucibles to maximize yield per batch, as the long dwell time limits the number of cycles per day.
The reliability of your sacrificial layer is directly determined by the consistency of the thermal treatment provided by the muffle furnace.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Target Temperature | 1100 °C | Drives solid-phase ion diffusion |
| Dwell Time | 10 Hours | Ensures complete stoichiometric reaction |
| Precursors | SrCO3 & Al2O3 | Primary raw materials for synthesis |
| Atmosphere | Atmospheric (Air) | Facilitates oxidation and decomposition |
| Critical Outcome | Phase Purity | Guarantees water-solubility of sacrificial layer |
Elevate Your Material Synthesis with KINTEK
Precision is non-negotiable when synthesizing high-performance sacrificial layers like Sr3Al2O6. KINTEK provides industry-leading thermal solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered for rigorous solid-phase reactions. Backed by expert R&D and advanced manufacturing, our lab high-temperature furnaces are fully customizable to meet your unique stoichiometric and thermal profile requirements.
Achieve phase-pure results every time. Contact us today to find your custom furnace solution!
Visual Guide
References
- Freestanding TiN‐Au Vertically Aligned Nanocomposite Thin Films for Flexible Plasmonic Hybrid Metasurfaces. DOI: 10.1002/admi.202500613
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How does a high-precision muffle furnace contribute to the evaluation of coatings? 1100°C Oxidation Test Insights
- What is a muffle furnace and how does it generally function? Master Indirect Heating for Pure Results
- Why was the muffle furnace initially developed? To Ensure Purity in High-Temperature Processing
- Why use an explosion-proof oven for silica aerogels? Essential Safety for High-Temp Ambient Pressure Drying
- What are some general-purpose uses of a muffle furnace? Essential for High-Purity Material Processing
- Why use a muffle furnace for AAS concrete testing? Achieve Precise Thermal Analysis for High-Temperature Performance
- What is the importance of programmable temperature control in a muffle furnace? Master g-C3N4 Synthesis Precision
- How should samples be handled when using a muffle furnace? Ensure Accurate and Safe Heat Treatment



















