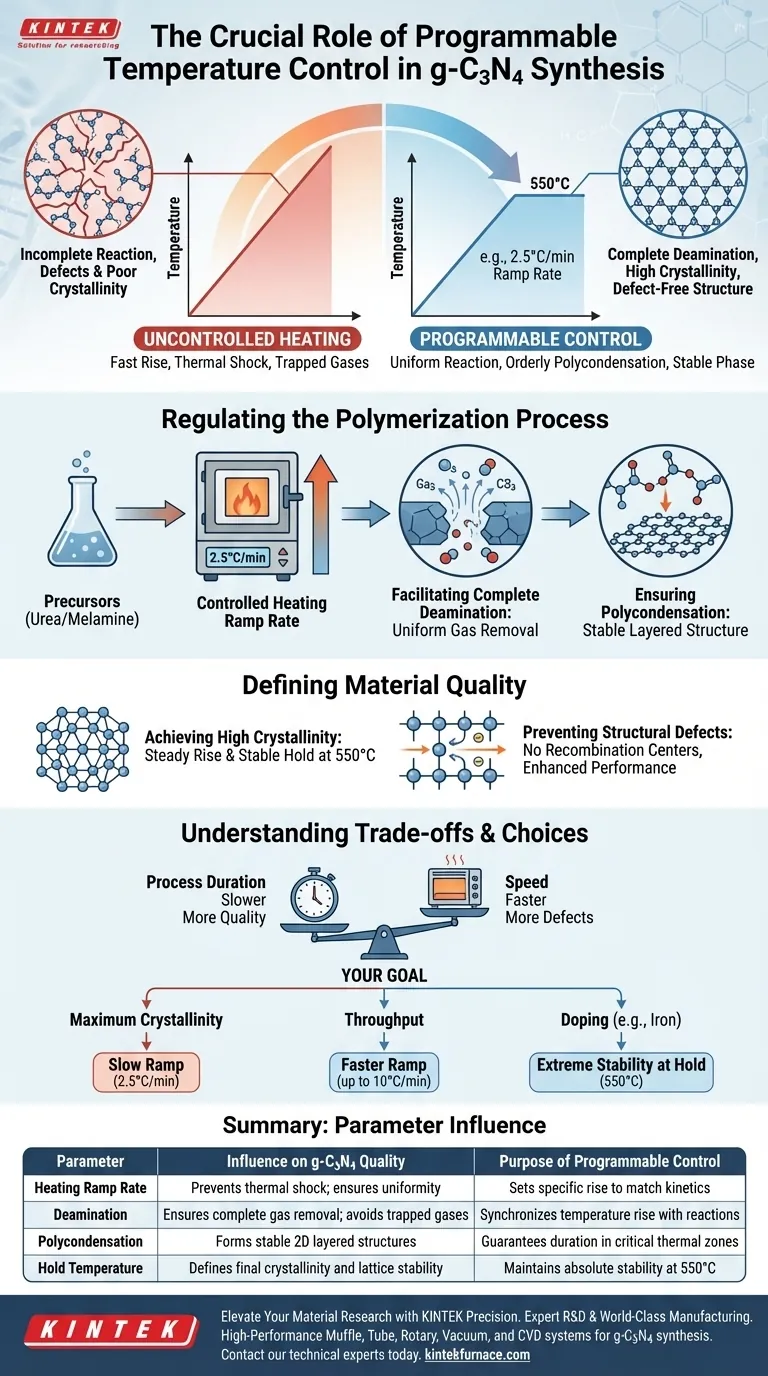
Precise thermal management is the deciding factor in the successful synthesis of graphitic carbon nitride (g-C3N4) via pyrolysis. A programmable temperature control feature allows you to strictly regulate the heating ramp rate—often as slow as 2.5°C per minute—to ensure raw materials like urea or melamine undergo complete polymerization. Without this granular control, the precursors may reach the target temperature of 550°C too quickly, resulting in incomplete deamination, structural defects, and poor crystallinity.
The core value of programmable control is its ability to synchronize the heating rate with the chemical reaction kinetics. By slowing the temperature rise, you ensure the orderly deamination and polycondensation of precursors, resulting in a stable, defect-free graphitic phase structure.
Regulating the Polymerization Process
Controlling the Heating Ramp Rate
The synthesis of g-C3N4 is not merely about reaching a final temperature; it is about how you get there.
A programmable furnace allows you to set a specific ramp rate, such as 2.5°C/min. This slow, controlled rise is critical because it prevents thermal shock to the precursor materials.
Facilitating Complete Deamination
For precursors like melamine or urea to transform into graphitic carbon nitride, they must undergo deamination (removal of amine groups).
If the temperature rises too rapidly, the outer layers of the bulk material may react before the inner layers, trapping gases. A programmable ramp ensures the reaction proceeds uniformly throughout the sample volume.
Ensuring Polycondensation
The transition from monomers to a polymerized structure requires a specific thermal energy profile.
Programmable control guarantees the material spends enough time in the critical temperature zones where polycondensation occurs. This ensures the precursors link together correctly to form the desired 2D layered structure before reaching the final holding temperature.
Defining Material Quality
Achieving High Crystallinity
The electronic properties of g-C3N4 are directly tied to its crystallinity.
A strictly controlled heating profile minimizes the formation of amorphous (disordered) regions. By maintaining a steady rise and a stable hold at 550°C, the furnace promotes the formation of a highly crystalline lattice.
Preventing Structural Defects
Rapid or uncontrolled heating is the primary cause of defects in the carbon nitride framework.
These defects act as recombination centers for charge carriers, effectively ruining the material's photocatalytic performance. Programmable control mitigates this by preventing the incomplete decomposition that occurs during fast temperature spikes.
Understanding the Trade-offs
Process Duration vs. Quality
The primary trade-off of using a highly controlled, slow ramp rate (e.g., 2.5°C to 5°C/min) is the total synthesis time.
A full cycle, including the ramp and the typical 4-hour hold time, can take significantly longer than uncontrolled heating. You are sacrificing speed for structural integrity and chemical purity.
Equipment Complexity and Cost
Furnaces with advanced programmable logic controllers (PLCs) are generally more expensive than simple set-point ovens.
They require more setup time to program the specific segments (ramp, soak, cool). However, for functional materials like semiconductors, this complexity is a requirement, not a luxury.
Making the Right Choice for Your Goal
Depending on your specific research or production needs, you should adjust your programming strategy accordingly:
- If your primary focus is Maximum Crystallinity: Set a slow ramp rate (approx. 2.5°C/min) to allow for the most orderly arrangement of the crystal lattice and minimal defects.
- If your primary focus is Throughput: Experiment with a faster ramp (up to 10°C/min), understanding that you may introduce some structural disorder or amorphous phases.
- If your primary focus is Doping (e.g., Iron-doped g-C3N4): Prioritize extreme stability at the holding temperature (550°C) to facilitate the incorporation of ions into the lattice.
Ultimate success in g-C3N4 synthesis relies on treating heat as a reagent that must be measured as precisely as your chemical precursors.
Summary Table:
| Parameter | Influence on g-C3N4 Quality | Purpose of Programmable Control |
|---|---|---|
| Heating Ramp Rate | Prevents thermal shock; ensures uniformity | Sets specific rise (e.g., 2.5°C/min) to match kinetics |
| Deamination | Ensures complete gas removal; avoids trapped gases | Synchronizes temperature rise with chemical reactions |
| Polycondensation | Forms stable 2D layered structures | Guarantees duration in critical thermal zones |
| Hold Temperature | Defines final crystallinity and lattice stability | Maintains absolute stability at 550°C for doping/purity |
Elevate Your Material Research with KINTEK Precision
Don't let uncontrolled thermal cycles compromise your material quality. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of g-C3N4 synthesis and advanced pyrolysis.
Our furnaces provide the granular, programmable control required for perfect crystallinity and defect-free structures. Whether you need a standard lab setup or a fully customizable high-temperature furnace tailored to your unique research needs, KINTEK delivers the reliability your work deserves.
Ready to optimize your synthesis results? Contact our technical experts today to find the perfect thermal solution.
Visual Guide
References
- Muhammad Saad, Mazloom Shah. Development of stable S-scheme 2D–2D g-C3N4/CdS nanoheterojunction arrays for enhanced visible light photomineralisation of nitrophenol priority water pollutants. DOI: 10.1038/s41598-024-52950-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What role does a muffle furnace play in g-C3N4 calcination? Master Precision Thermal Polycondensation
- Why is a box resistance furnace utilized for the homogenization annealing of alloy micro-wires? Key Benefits Explained
- What industries still require traditional retort-based Muffle Furnace designs? Essential for High-Temperature Atmospheric Integrity
- How does the insulated chamber of a muffle furnace function? Unlock Precise, Contamination-Free Heating
- What are the main structural components of a muffle furnace? Discover the Engineered System for Contamination-Free Heating
- What should be considered regarding the working temperature of a muffle furnace? Ensure Precision and Longevity for Your Lab
- What is the function of a laboratory high-temperature furnace in cook-off synthesis? A Precise Thermal Initiator
- What is the primary function of a muffle furnace in PI microfibers? Enhance Polyimide Thermal Imidization



















