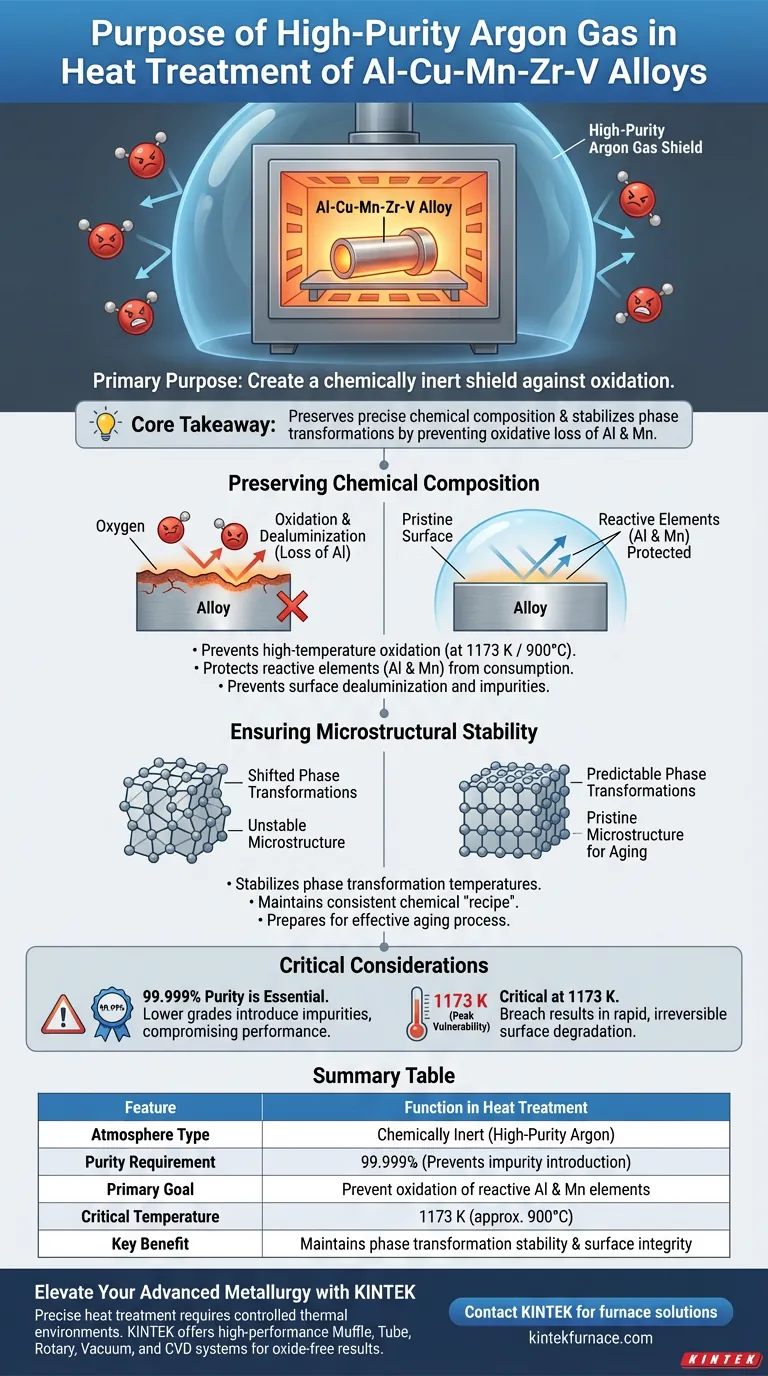
The primary purpose of using high-purity argon gas is to create a chemically inert shield against oxidation. During the critical high-temperature stages of heat treating Al-Cu-Mn-Zr-V alloys, specifically solution treatment, argon displaces the air surrounding the metal. This prevents oxygen from reacting with the aluminum matrix and ensures the surface chemistry remains intact.
Core Takeaway The essential function of high-purity argon is to preserve the alloy's precise chemical composition by preventing the oxidative loss of reactive elements like aluminum and manganese. This protection stabilizes phase transformation temperatures and ensures the microstructure is pristine for subsequent aging processes.
Preserving Chemical Composition
The Threat of High-Temperature Oxidation
When Al-Cu-Mn-Zr-V alloys are subjected to extreme heat (such as 1173 K or approx. 900°C), they become highly reactive. Without a protective atmosphere, oxygen in the air attacks the metal surface.
Protecting Reactive Elements (Al and Mn)
Aluminum and manganese are chemically active elements that oxidize easily at high temperatures. High-purity argon creates a barrier that prevents these specific elements from being consumed by oxygen.
Preventing Surface Dealuminization
If the aluminum matrix reacts with oxygen, the surface suffers from dealuminization (the loss of aluminum). This degrades the material's integrity and introduces unwanted impurities into the surface layers.
Ensuring Microstructural Stability
Stabilizing Phase Transformations
The precise balance of elements determines the temperatures at which the alloy changes phases. If components like manganese are lost to oxidation, the phase transformation temperatures will shift. Argon ensures the chemical "recipe" stays constant, keeping the alloy's physical behavior predictable.
Preparing for the Aging Process
The solution treatment stage sets the foundation for the subsequent aging process. By maintaining a high-quality microstructure free of oxides, argon ensures the alloy can respond correctly to aging, developing the intended mechanical strength and performance.
Critical Considerations and Pitfalls
The Necessity of 99.999% Purity
Not all argon is created equal. For these sensitive alloys, 99.999% purity is required. Using lower-grade argon can inadvertently introduce impurities, negating the protective benefits and compromising the alloy's low-temperature performance.
Vulnerability at 1173 K
The specific temperature of 1173 K used during solid solution treatment represents a peak vulnerability point. Any breach in the argon atmosphere at this temperature will result in rapid, irreversible surface degradation.
Making the Right Choice for Your Goal
To maximize the effectiveness of your heat treatment process, consider these priorities:
- If your primary focus is compositional accuracy: Ensure your gas supply is certified to 99.999% purity to prevent trace element loss in the Mn and Al matrix.
- If your primary focus is mechanical reliability: Monitor the furnace atmosphere continuously to prevent phase transformation shifts that could weaken the alloy's final structure.
By strictly controlling the atmosphere with high-purity argon, you transform a volatile heating process into a precise engineering operation.
Summary Table:
| Feature | Function in Heat Treatment |
|---|---|
| Atmosphere Type | Chemically inert (High-purity Argon) |
| Purity Requirement | 99.999% to prevent impurity introduction |
| Primary Goal | Prevent oxidation of reactive Al and Mn elements |
| Critical Temperature | 1173 K (approx. 900°C) |
| Key Benefit | Maintains phase transformation stability and surface integrity |
Elevate Your Advanced Metallurgy with KINTEK
Precise heat treatment of sensitive alloys like Al-Cu-Mn-Zr-V requires more than just high-purity gas—it requires a controlled thermal environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain pristine atmospheric conditions. Whether you are conducting solution treatments or complex aging processes, our customizable lab high-temperature furnaces ensure your microstructures remain oxide-free and predictable.
Ready to achieve superior material performance? Contact KINTEK today to find the perfect furnace solution for your specialized research and production needs.
Visual Guide
References
- Dequan Shi, Kaijiao Kang. Effect of Electro-Pulse on Microstructure of Al-Cu-Mn-Zr-V Alloy during Aging Treatment and Mechanism Analysis. DOI: 10.3390/met14060648
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
People Also Ask
- Why is a high-temperature furnace with gas flow control necessary for Li6MnO4 precursors? Achieve Precise Synthesis
- Why is a furnace with programmed temperature control required for catalyst regeneration? Ensure Catalyst Stability
- What are the advantages of using ultrasonic spray pyrolysis for metal powder? Achieve High Purity & Sphericity
- Why is a constant temperature drying oven set to 60°C for 24 hours? Optimizing Sr4Al6O12SO4 Powder Quality
- How does heat treatment affect the TPU encapsulation layer? Optimize Flexible Sensor Durability & Bonding
- How does extending the duration of high-temperature constant phase affect iron grain growth? Maximize Zinc Extraction
- Why is high-purity argon gas required for MTO synthesis? Ensure Stability in Rhenium Organometallic Catalysis
- What are the advantages of using a stainless steel fixed bed reactor for biochar pyrolysis? Unlock Precise Lab Results



















