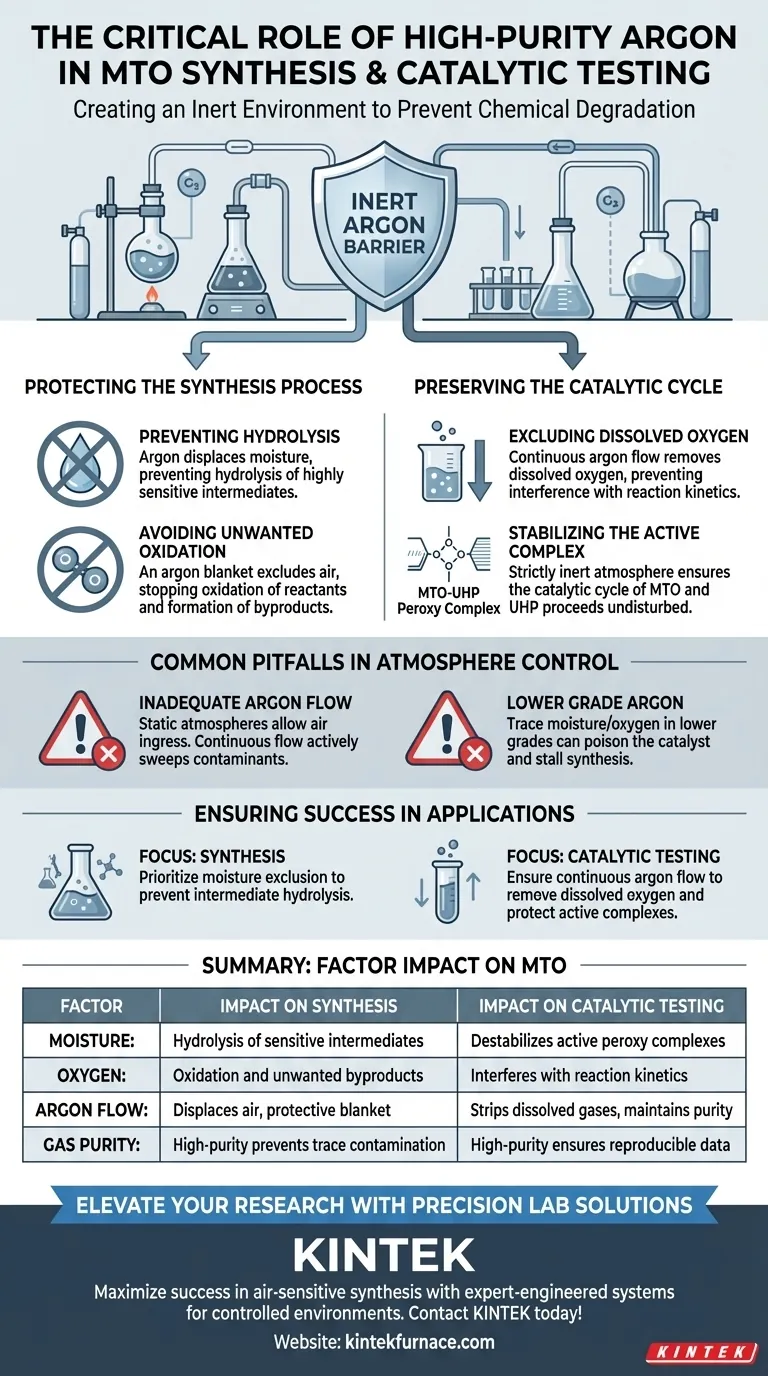
High-purity argon gas is strictly required to create an inert environment that prevents chemical degradation. Methyltrioxorhenium (MTO) and its synthetic intermediates are organometallic compounds highly susceptible to moisture and air. Argon acts as a barrier, preventing hydrolysis and oxidation that would otherwise ruin the synthesis or skew catalytic testing results.
The use of argon is not merely a precaution; it is a fundamental requirement to exclude moisture and dissolved oxygen, which destabilize reactive intermediates and disrupt the MTO-UHP catalytic cycle.

Protecting the Synthesis Process
The synthesis of organometallic rhenium compounds involves delicate chemical pathways. The presence of standard atmospheric components can cause immediate failure of the reaction.
Preventing Hydrolysis
During the initial creation of MTO, various chemical intermediates are formed. These intermediates are highly sensitive to moisture.
If water vapor is present, these compounds undergo hydrolysis, breaking down before they can be converted into the final MTO product. High-purity argon displaces this moisture, preserving the structural integrity of the intermediates.
Avoiding Unwanted Oxidation
In addition to moisture, oxygen is a primary threat during synthesis. The intermediates involved are prone to reacting with atmospheric oxygen.
An argon blanket effectively excludes air, preventing oxidation that would otherwise degrade the reactants or produce unwanted byproducts.
Preserving the Catalytic Cycle
Once MTO is synthesized and used for testing, the need for a protective atmosphere remains critical. The validity of catalytic testing data depends on the stability of the reaction environment.
Excluding Dissolved Oxygen
During catalytic testing, MTO is often used in conjunction with urea hydrogen peroxide (UHP). It is vital to maintain an argon flow through the reaction vessel to remove dissolved oxygen.
If oxygen remains dissolved in the solvent, it can interfere with the reaction kinetics, leading to inaccurate performance data.
Stabilizing the Active Complex
The core of the catalytic process involves the formation of active peroxy complexes between MTO and UHP. This is the "engine" of the catalysis.
Environmental contaminants, such as moisture or air, can disturb this cycle. By maintaining a strictly inert argon atmosphere, you ensure the catalytic cycle proceeds undisturbed, yielding reliable and reproducible results.
Common Pitfalls in Atmosphere Control
While using argon is standard, the method of application matters. Understanding the risks of inadequate control is essential for successful experimentation.
The Necessity of Flow
Simply filling a vessel with argon is often insufficient. The reference highlights the importance of maintaining an argon flow.
Static atmospheres may eventually allow air ingress or fail to fully strip dissolved gases from the solution. A continuous flow actively sweeps away contaminants.
The Requirement for High Purity
Not all argon is created equal. The requirement is specifically for high-purity argon.
Lower grades of argon may contain trace amounts of moisture or oxygen. Given the sensitivity of MTO intermediates, even these trace impurities can be enough to poison the catalyst or stall the synthesis.
Ensuring Success in MTO Applications
The application of argon must be tailored to the specific stage of your work to ensure chemical stability.
- If your primary focus is Synthesis: Prioritize the exclusion of moisture to prevent the hydrolysis of sensitive intermediates.
- If your primary focus is Catalytic Testing: Ensure a continuous argon flow to remove dissolved oxygen and protect the formation of active peroxy complexes.
Consistency in your inert atmosphere leads to consistency in your chemical results.
Summary Table:
| Factor | Impact on MTO Synthesis | Impact on Catalytic Testing |
|---|---|---|
| Moisture | Causes hydrolysis of sensitive intermediates | Destabilizes active peroxy complexes |
| Oxygen | Leads to oxidation and unwanted byproducts | Interferes with reaction kinetics |
| Argon Flow | Displaces air and creates a protective blanket | Strips dissolved gases and maintains purity |
| Gas Purity | High-purity prevents trace contamination | High-purity ensures reproducible data |
Elevate Your Research with Precision Lab Solutions
Maximize the success of your air-sensitive organometallic synthesis with KINTEK. Whether you are working with Rhenium compounds or other delicate catalysts, our expert-engineered systems provide the controlled environments necessary for reproducible results.
Why choose KINTEK?
- Customizable High-Temp Furnaces: Including Muffle, Tube, Rotary, and Vacuum systems tailored to your inert gas requirements.
- Expert R&D Support: Backed by industry-leading manufacturing to support your unique laboratory needs.
- Superior Atmosphere Control: Designed to eliminate contaminants like moisture and oxygen.
Contact KINTEK today to discuss how our customizable high-temperature solutions can optimize your synthesis and catalytic testing workflows!
Visual Guide
References
- Joanna Malarz, Katarzyna Leszczyńska-Sejda. Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials. DOI: 10.3390/cryst15080717
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What is the role of industrial electric drying ovens in FDSSC titanium photoanode treatment? Enhance Solar Efficiency
- How does a vacuum pressure infiltration system contribute to Diamond/Cu composite green bodies? Achieve 60% Density
- Why is a high-pressure digestion tank essential for ZnO/rGO hydrothermal synthesis? Achieve superior interfacial coupling
- How do industrial heating furnaces and rolling mills optimize Invar 36? Master Thermal Stability and Deformation
- What is the significance of using a laboratory electric furnace for the quenching and tempering of hull steel? Achieve Precise Microstructure Control
- What advantages does a vacuum drying oven offer? Superior Chemical Stability & Efficient Dehydration
- What are the advantages of using a batch furnace? Achieve Unmatched Process Flexibility and Precision
- What function does high-purity argon gas serve in BPEA PVT preparation? Ensure High-Quality Crystal Growth













