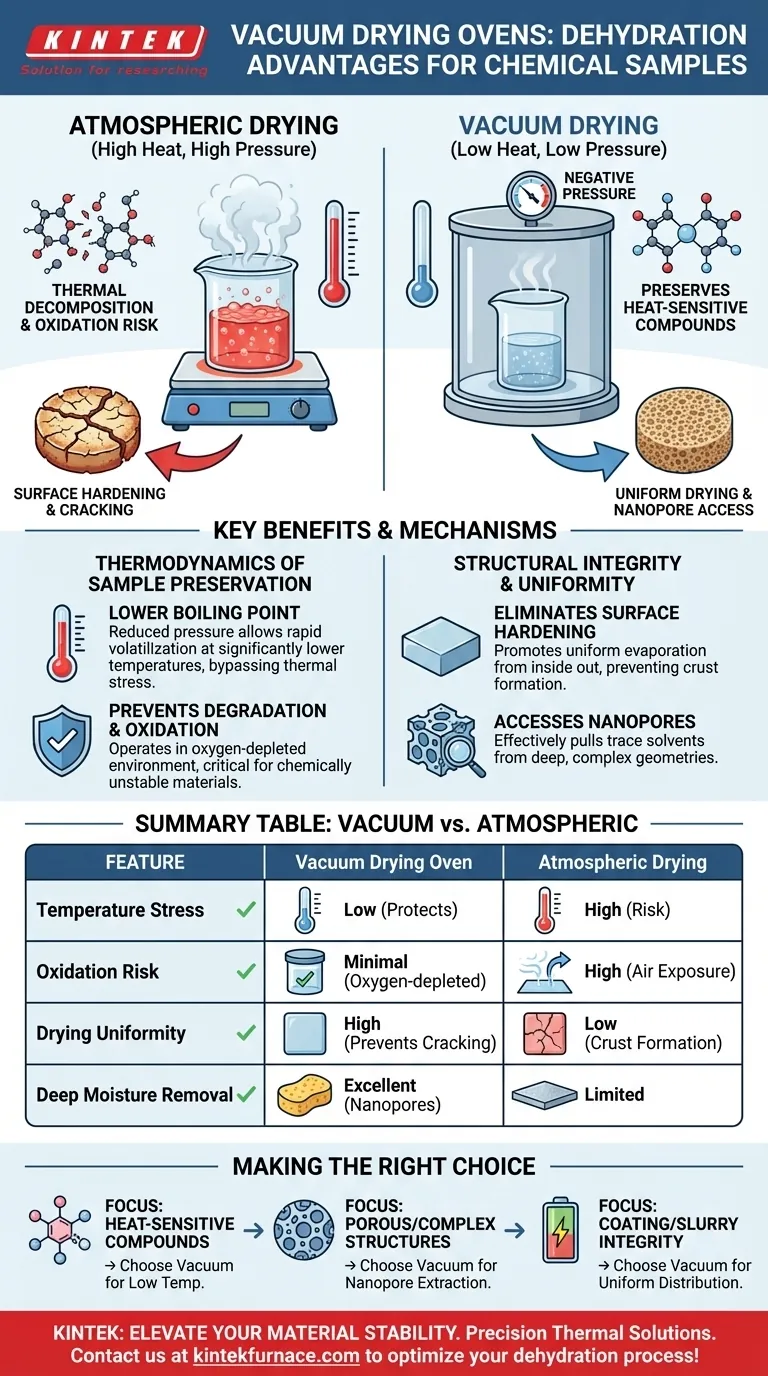
The primary advantage of a vacuum drying oven is its ability to lower the boiling point of solvents by reducing the pressure within the chamber. This allows for rapid dehydration at significantly lower temperatures compared to atmospheric drying, effectively preventing the thermal decomposition or oxidation of heat-sensitive chemical substances.
By manipulating pressure rather than just temperature, vacuum drying bypasses the physical limitations of atmospheric heating. It ensures complete solvent removal without compromising the chemical stability or structural integrity of delicate samples.

The Thermodynamics of Sample Preservation
Lowering the Boiling Point
The fundamental mechanism of a vacuum oven is the creation of a negative pressure environment. As pressure drops, the energy required for solvents to transition from liquid to gas decreases.
This means water or organic solvents can volatilize rapidly at temperatures far below their standard boiling points. You can achieve effective drying without subjecting your sample to aggressive thermal stress.
Preventing Thermal Degradation and Oxidation
For chemically unstable or heat-sensitive materials, high temperatures are destructive. Vacuum drying mitigates this risk by operating in a cooler, oxygen-depleted environment.
This is critical for materials prone to oxidation, such as specific metal oxides or organic cores. By removing air and heat, you ensure the material remains chemically pure during the drying process.
Structural Integrity and Uniformity
Eliminating Surface Hardening
A common failure mode in atmospheric drying is "surface hardening" or skinning. This occurs when the outer layer of a sample dries and hardens too quickly, trapping moisture or solvents deep inside the material.
Vacuum drying promotes uniform evaporation from the inside out. This prevents the formation of a hard crust, ensuring that the sample is thoroughly dried throughout its volume.
Accessing Nanopores and Complex Geometries
Atmospheric drying often struggles to remove solvents trapped in microscopic structures. The vacuum environment effectively pulls trace solvents out of deep nanopores and complex geometries.
This capability is essential for preparing core-shell materials or porous structures that must be perfectly dry before undergoing subsequent high-temperature processes like calcination.
Understanding the Trade-offs
Equipment Complexity vs. Sample Quality
While atmospheric drying is mechanically simpler, it lacks the precision required for advanced materials. The trade-off for the superior results of a vacuum oven is the need for a sealed system capable of maintaining consistent negative pressure.
The Risk of Uneven Atmospheric Drying
Attempting to dehydrate slurries or coated materials (such as battery anodes) in an atmospheric oven often leads to cracking or uneven binder distribution.
Vacuum drying avoids this by controlling the volatilization rate. It ensures binders remain uniformly distributed between active materials, which directly correlates to the mechanical stability of the final product.
Making the Right Choice for Your Goal
To determine if vacuum drying is necessary for your specific application, consider the physical properties of your sample.
- If your primary focus is preserving heat-sensitive compounds: Vacuum drying is mandatory to prevent decomposition and oxidation by keeping process temperatures low.
- If your primary focus is drying porous or complex structures: The vacuum environment is required to extract trace solvents from nanopores that atmospheric heat cannot reach.
- If your primary focus is coating or slurry integrity: Use vacuum drying to prevent surface cracking and ensure a uniform distribution of internal components.
Mastering vacuum drying allows you to decouple temperature from evaporation, giving you total control over the dehydration process.
Summary Table:
| Feature | Vacuum Drying Oven | Atmospheric Drying |
|---|---|---|
| Temperature Stress | Low (protects heat-sensitive materials) | High (risk of thermal decomposition) |
| Boiling Point | Reduced via negative pressure | Standard (requires more heat) |
| Oxidation Risk | Minimal (oxygen-depleted environment) | High (exposure to air at high heat) |
| Drying Uniformity | High (prevents surface hardening/cracking) | Low (risk of crust formation) |
| Deep Moisture Removal | Excellent for nanopores/complex shapes | Limited for internal structures |
Elevate Your Material Stability with KINTEK
Don’t compromise the integrity of your heat-sensitive chemical samples. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum drying systems and customizable lab high-temp furnaces (Muffle, Tube, Rotary, CVD) designed for your unique research needs. Our precision equipment ensures complete solvent removal while preventing oxidation and structural damage.
Ready to optimize your dehydration process? Contact KINTEK today to find the perfect thermal solution for your laboratory!
Visual Guide
References
- Xiaoyan Xiong, Tao Jin. Ta/Organo‐In Nanomaterials for Low‐Power or Room Temperature Triethylamine Response. DOI: 10.1002/slct.202405960
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is temperature gradient management necessary for high-temperature impedance measurements? Master Thermal Precision
- Why is a laboratory vacuum evaporation system essential for the preparation of electrodes in high-performance solar cells?
- What causes the increase in specific gravity of Moso Bamboo? Master Cellular Densification in Heat Treatment
- What type of furnaces are commonly used for sintering? Choose the Right Furnace for Your Process
- What are the equipment requirements for high-temperature furnaces during magnetic biochar synthesis? Find the key specs.
- What is a batch furnace and how does it operate? Master Precision Heat Treatment for Diverse Applications
- Why are graphite molds preheated to 800 °C for Invar 36 casting? Unlock High-Quality Ingot Production
- What role does a high-temperature sintering furnace play in lead-free piezoelectric ceramics? Optimizing Performance



















