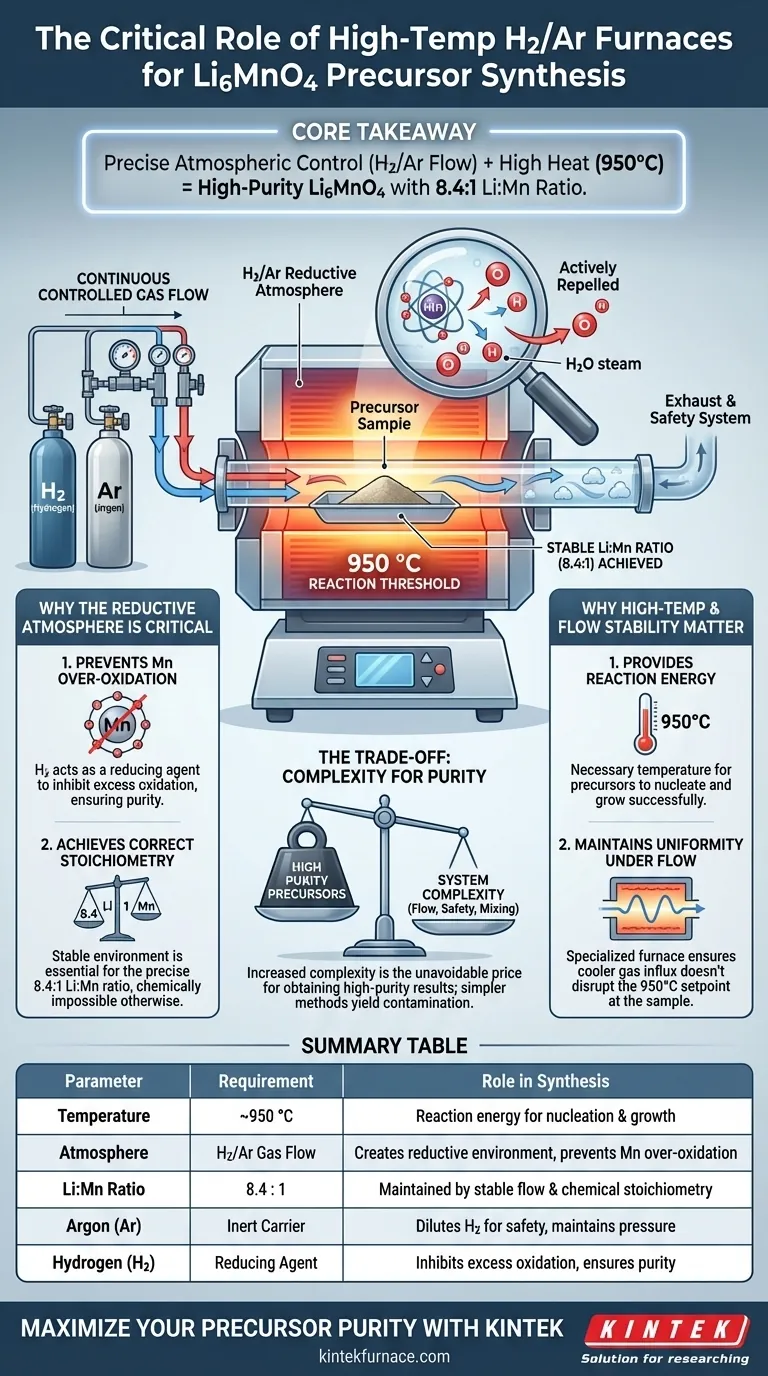
Precise atmospheric control is the critical factor in synthesizing Li6MnO4 precursors. To prepare these precursors successfully, you must use a high-temperature tube furnace capable of maintaining a continuous flow of hydrogen (H2) and argon (Ar) gas at approximately 950 °C. This specific setup is required to create a stable reductive environment that prevents the manganese from reacting with excess oxygen.
Core Takeaway The synthesis of Li6MnO4 requires a delicate balance of high heat and a reductive atmosphere. Without a continuous H2/Ar flow, manganese over-oxidizes at 950 °C, which destroys the required 8.4:1 Lithium-to-Manganese ratio and compromises the purity of the material.

The Critical Role of the Reductive Atmosphere
Preventing Manganese Over-Oxidation
At high synthesis temperatures, manganese is highly susceptible to bonding with oxygen. If left unchecked, this leads to over-oxidation, resulting in impurities rather than the desired precursor. The hydrogen component of the gas flow acts as a reducing agent to actively inhibit this excess oxidation.
Achieving the Correct Stoichiometry
The target formulation requires a specific Lithium-to-Manganese (Li:Mn) ratio of 8.4:1. Achieving this exact ratio is chemically impossible if the manganese oxidation state fluctuates. The continuous gas flow stabilizes the reaction environment, ensuring the chemical inputs combine in the correct proportions.
The Function of Argon
Argon acts as an inert carrier gas within the mixture. It helps dilute the hydrogen to safe, manageable levels while maintaining positive pressure in the furnace. This ensures a uniform flow environment over the sample material.
Thermal Requirements for Synthesis
Reaching the Reaction Threshold
The chemical reaction required to form Li6MnO4 precursors occurs at approximately 950 °C. This temperature provides the necessary energy for the precursors to nucleate and grow. Below this threshold, the reaction may remain incomplete or fail to initiate entirely.
Stability Under Flow Conditions
A specialized tube furnace is necessary to maintain this high temperature while gas is moving through the chamber. Standard furnaces may struggle to maintain thermal uniformity when subjected to continuous gas exchange. The equipment must ensure that the introduction of cooler gas does not disrupt the 950 °C setpoint at the sample site.
Understanding the Trade-offs
Complexity vs. Purity
Introducing a gas flow system significantly increases the complexity of the experimental setup compared to static air calcination. You must manage flow rates, gas mixing ratios, and exhaust safety. However, this complexity is the unavoidable price for obtaining high-purity precursors; simpler methods will yield contaminated results.
Sensitivity to Fluctuations
The process is highly sensitive to interruptions in gas flow. Even a momentary lapse in the reductive atmosphere at 950 °C can ruin the batch by allowing immediate oxidation. Therefore, the equipment must offer precise, uninterrupted control rather than manual or intermittent adjustments.
Making the Right Choice for Your Goal
To ensure successful synthesis, you must prioritize equipment capabilities based on your specific quality metrics.
- If your primary focus is Chemical Purity: Ensure your furnace controller offers precise, automated mass flow controllers to keep the H2/Ar ratio constant throughout the entire 950 °C hold time.
- If your primary focus is Stoichiometric Accuracy: Verify that the furnace maintains excellent thermal uniformity (±5 °C) along the tube length to ensure the 8.4:1 ratio is achieved across the entire sample volume.
The combination of a reductive H2/Ar atmosphere and stable high heat is not optional; it is the fundamental requirement for controlling manganese chemistry in this synthesis.
Summary Table:
| Parameter | Requirement | Role in Synthesis |
|---|---|---|
| Temperature | ~950 °C | Provides reaction energy for nucleation and growth |
| Atmosphere | H2/Ar Gas Flow | Creates reductive environment to prevent Mn over-oxidation |
| Li:Mn Ratio | 8.4 : 1 | Maintained by stable gas flow and chemical stoichiometry |
| Argon Function | Inert Carrier | Dilutes H2 for safety and maintains chamber pressure |
| Hydrogen Role | Reducing Agent | Inhibits excess oxidation to ensure precursor purity |
Maximize Your Precursor Purity with KINTEK
Precise atmospheric control is the difference between high-purity Li6MnO4 and contaminated batches. KINTEK provides advanced high-temperature Tube, Vacuum, and CVD systems specifically designed for sensitive synthesis processes. Backed by expert R&D and manufacturing, our furnaces offer the precise gas flow control and thermal uniformity (±5°C) required to maintain your exact 8.4:1 stoichiometry.
Ready to elevate your material research? Contact KINTEK today to discuss our customizable lab solutions for your unique high-temp needs.
Visual Guide
References
- Venkata Sai Avvaru, Haegyeom Kim. Alternative Solid‐State Synthesis Route for Highly Fluorinated Disordered Rock‐Salt Cathode Materials for High‐Energy Lithium‐Ion Batteries. DOI: 10.1002/aenm.202500492
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the advantages of using a vacuum drying oven for purifying zinc oxide nanoparticles? Superior Material Quality
- What is the impact of using a vacuum drying oven on CDI electrodes? Optimize Stability and Conductivity
- What is the temperature of a graphite furnace? Unlock Extreme Heat Up to 3000°C
- Why are high-purity copper foils used as support substrates in phase equilibrium experiments with low SiO2 content?
- Why is the mechanical mixing of precursor powders necessary for ITO thin films? Guide to Precision Growth
- Why is the calcination process essential for Fe3O4/CeO2 and NiO/Ni@C? Control Phase Identity and Conductivity
- How does a single-action hydraulic press ensure the quality of green compacts? Key Factors for Aluminum Composites
- Why is a vacuum desiccator used for the preservation of extracted fruit peel extracts? Protect Bioactive Compounds



















