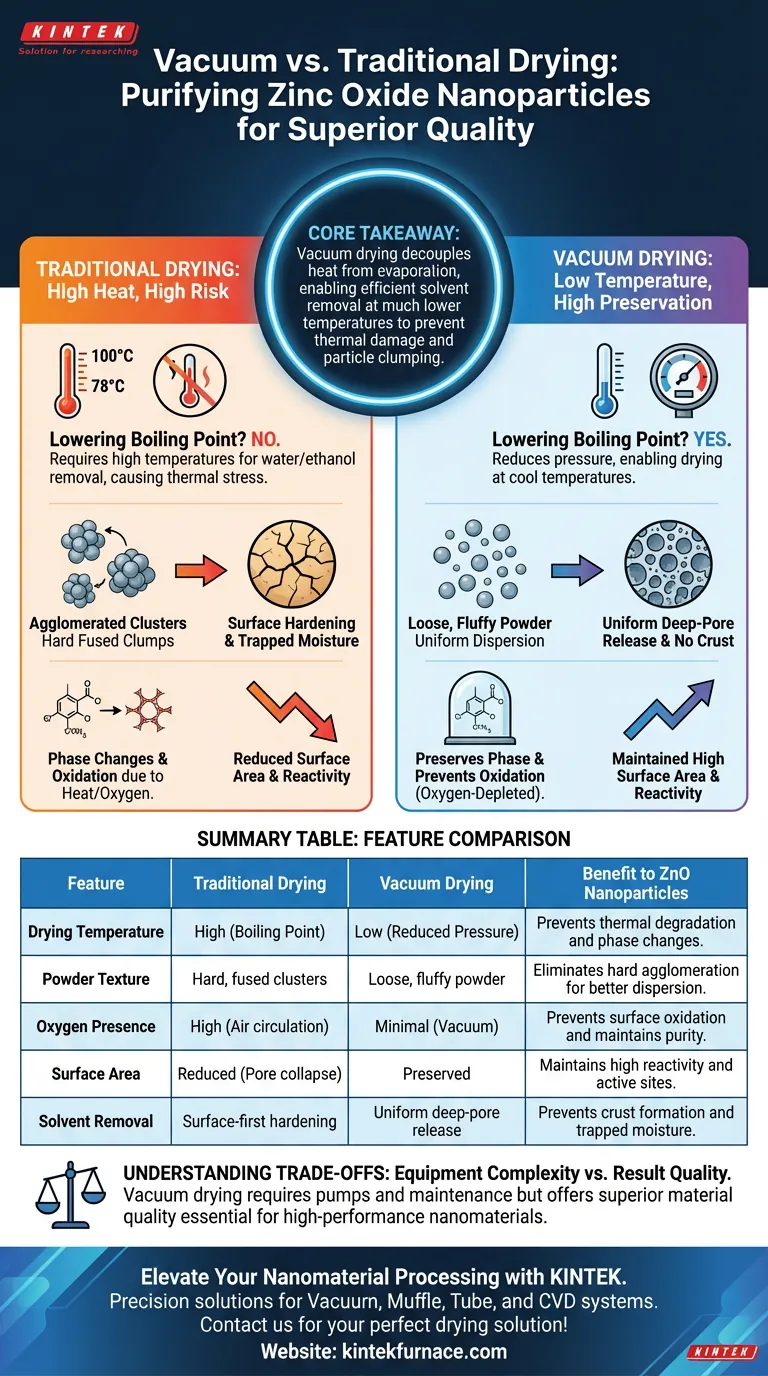
The primary advantage of using a vacuum drying oven for zinc oxide nanoparticles is the ability to remove solvents at significantly lower temperatures by reducing the environmental pressure. This protects the nanomaterials from the thermal stress inherent in traditional drying methods, ensuring the removal of residual ethanol and water without compromising the particle's structure.
Core Takeaway Vacuum drying decouples heat from evaporation, allowing for efficient solvent removal without the high temperatures that cause phase changes or particle clumping. This method is essential for producing loose, highly active zinc oxide powder rather than hard, agglomerated clusters.

Preserving Nanostructure Integrity
Lowering the Boiling Point
In a traditional oven, you must heat solvents like water or ethanol to their standard boiling points (100°C and 78°C, respectively) to remove them effectively.
A vacuum oven lowers the pressure within the chamber, which depresses the boiling point of these solvents. This allows you to dry the zinc oxide at much cooler temperatures, preventing the thermal degradation that occurs in high-heat environments.
Preventing Phase Changes
Zinc oxide nanoparticles are sensitive to thermal history; excessive heat can alter their crystalline phase.
By drying under vacuum, you avoid the high thermal energy required by atmospheric drying. This ensures the material retains its intended phase and does not undergo unwanted structural transformations during the purification process.
Combatting Agglomeration
Achieving Loose Powder Consistency
One of the greatest challenges in drying nanoparticles is "hard agglomeration," where particles fuse together into unusable clumps.
Traditional drying often causes rapid evaporation at the surface, creating a crust that traps moisture inside and pulls particles together via strong capillary forces. Vacuum drying promotes a more uniform release of solvents, resulting in a loose, fluffy powder that is easy to disperse in subsequent applications.
Eliminating Surface Hardening
In atmospheric conditions, drying can lead to surface hardening, where the outer layer dries before the core.
The vacuum environment prevents this by ensuring that solvents can escape from deep within the material pores even at low temperatures. This prevents the physical structural collapse of the nanoparticles and maintains the material's accessible surface area.
Enhancing Material Activity
Maintaining High Surface Reactivity
The effectiveness of zinc oxide nanoparticles often depends on their specific surface area and active sites.
High-temperature drying in standard ovens can induce oxidation or reduce surface area through densification. Vacuum drying preserves the high activity of the powder, ensuring it remains chemically reactive for its end-use application.
Preventing Oxidation
While standard ovens circulate air (and therefore oxygen), a vacuum oven removes air from the chamber.
This oxygen-depleted environment is critical for preventing thermal oxidation on the surface of the nanoparticles. It ensures that the chemical purity of the zinc oxide is maintained throughout the drying cycle.
Understanding the Trade-offs
Equipment Complexity vs. Result Quality
While vacuum drying offers superior material quality, it introduces operational complexity compared to simple forced-air ovens.
You must manage vacuum pumps and ensure airtight seals, which requires more maintenance than a standard thermostatic oven. However, for high-performance nanomaterials, the gain in particle quality and consistency invariably outweighs the increased equipment requirements.
Making the Right Choice for Your Goal
To ensure you select the correct drying protocol for your specific requirements, consider the following:
- If your primary focus is maximizing surface area: Choose vacuum drying to prevent pore collapse and liquid bridge forces that lead to hard agglomeration.
- If your primary focus is crystalline purity: Rely on vacuum drying to remove solvents below the threshold temperature that would trigger phase changes or oxidation.
- If your primary focus is preventing agglomeration: Use vacuum drying to ensure the final product remains a loose powder rather than forming hard, fused clusters.
Vacuum drying is not just a drying method; it is a preservation technique that ensures the zinc oxide nanoparticles you synthesize are the same ones you recover.
Summary Table:
| Feature | Traditional Drying | Vacuum Drying | Benefit to ZnO Nanoparticles |
|---|---|---|---|
| Drying Temperature | High (Boiling Point) | Low (Reduced Pressure) | Prevents thermal degradation and phase changes. |
| Powder Texture | Hard, fused clusters | Loose, fluffy powder | Eliminates hard agglomeration for better dispersion. |
| Oxygen Presence | High (Air circulation) | Minimal (Vacuum) | Prevents surface oxidation and maintains purity. |
| Surface Area | Reduced (Pore collapse) | Preserved | Maintains high reactivity and active sites. |
| Solvent Removal | Surface-first hardening | Uniform deep-pore release | Prevents crust formation and trapped moisture. |
Elevate Your Nanomaterial Processing with KINTEK
Don't compromise the integrity of your zinc oxide nanoparticles with outdated drying methods. At KINTEK, we understand that precision is paramount in nanomaterial synthesis. Backed by expert R&D and world-class manufacturing, we provide high-performance Vacuum, Muffle, Tube, and CVD systems tailored for researchers and industrial manufacturers.
Whether you need to eliminate hard agglomeration or preserve crystalline purity, our customizable lab high-temperature furnaces ensure your materials retain their intended properties. Contact us today to find the perfect drying solution for your lab!
Visual Guide
References
- Kamilia Madi, Abdeltif Amrane. Green Fabrication of ZnO Nanoparticles and ZnO/rGO Nanocomposites from Algerian Date Syrup Extract: Synthesis, Characterization, and Augmented Photocatalytic Efficiency in Methylene Blue Degradation. DOI: 10.3390/catal14010062
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What is the primary design purpose of industrial electric furnaces for SA-178 Gr A steel? Ensure Pipe End Reliability
- What is the purpose of introducing high-purity argon gas into an immersed probe? Enhance Melt Analysis Accuracy
- How does the heat treatment of NC6 (1.2063) tool steel affect its performance? Key SPIF Tool Optimization
- What is the role of temperature control in MCM-41 synthesis? Master Precision Pore Engineering
- Why is a standard constant temperature and humidity curing box used for magnesium slag mortar? Key Pre-treatment Facts
- Why is a constant temperature and humidity curing chamber essential for geopolymerization? Ensure Structural Strength
- How does the use of a stainless steel high-pressure autoclave affect ZnS/CeO2@CNT formation? Optimize Catalyst Growth
- What is the purpose of using a vacuum drying oven for mineral powders? Optimize Polymer Bonding and Density



















