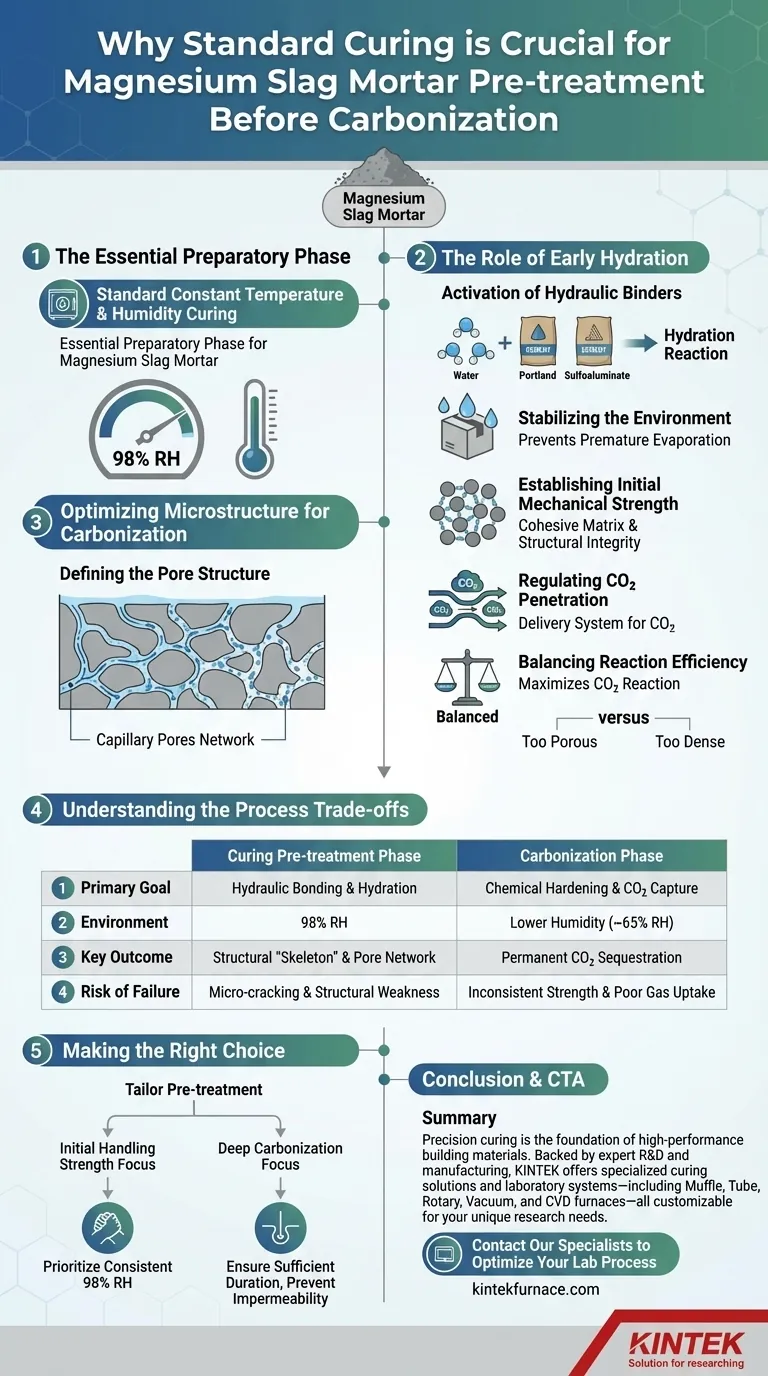
Standard constant temperature and humidity curing is the essential preparatory phase for magnesium slag mortar. This process utilizes a sealed box to maintain a high-humidity environment (typically 98% RH) which drives the initial hydration of hydraulic components like Portland or sulfoaluminate cement. This step is strictly required to establish the material's physical framework before it is exposed to carbon dioxide.
While the carbonization stage drives the final chemical hardening and CO2 sequestration, the pre-treatment phase constructs the physical "skeleton" of the material. Without the initial strength and specific pore structure developed during this standard curing, the subsequent carbonization process would lack the necessary matrix to function efficiently.

The Role of Early Hydration
Activation of Hydraulic Binders
Magnesium slag mortar typically contains hydraulic materials, such as Portland cement or sulfoaluminate cement. These materials require sufficient moisture to initiate their chemical reaction, known as hydration.
Stabilizing the Environment
The curing box ensures a stable, high-humidity atmosphere. This prevents the premature evaporation of mixing water, ensuring that the water remains available to react with the cementitious binders rather than drying out.
Establishing Initial Mechanical Strength
Before the mortar can withstand the pressures and chemical changes of the carbonization chamber, it must have a baseline level of structural integrity. Standard curing allows the cement hydration products to bond the magnesium slag particles together, creating a cohesive matrix.
Optimizing Microstructure for Carbonization
Defining the Pore Structure
The most critical function of pre-treatment is the regulation of the material's pore structure. As the cement hydrates, it fills certain voids within the matrix, creating a network of capillary pores.
Regulating CO2 Penetration
The pore network established during pre-treatment acts as the delivery system for the next stage. It dictates the penetration paths for carbon dioxide.
Balancing Reaction Efficiency
If the material is too porous, CO2 may pass through without reacting fully. If it is too dense, CO2 cannot penetrate deep into the core. Pre-treatment establishes the optimal balance to maximize the reaction between CO2 and minerals like dicalcium silicate later on.
Understanding the Process Trade-offs
Hydration vs. Carbonization Requirements
It is vital to distinguish the needs of the two stages. Pre-treatment requires high humidity (approx. 98%) to promote hydraulic bonding. In contrast, the subsequent carbonization stage often requires lower humidity (e.g., 65%) to facilitate gas diffusion.
The Risk of Skipping Pre-treatment
Attempting to carbonize uncured mortar can lead to structural failure. Without the initial hydraulic bonds, the matrix may be too weak to support the rapid formation of carbonates, potentially leading to micro-cracking or surface dusting.
The Risk of Improper Curing
If the pre-treatment environment fluctuates in temperature or humidity, the pore structure will form unevenly. This leads to inconsistent CO2 uptake in the final product, resulting in variable strength and sequestration performance.
Making the Right Choice for Your Goal
To optimize the production of magnesium slag mortar, you must tailor the pre-treatment phase to your specific performance targets.
- If your primary focus is Initial Handling Strength: Prioritize a consistent 98% RH environment to maximize the hydration of the Portland or sulfoaluminate cement components.
- If your primary focus is Deep Carbonization: Ensure the pre-treatment duration is sufficient to set the matrix but not so long that the pores become impermeable to gas diffusion.
The success of permanent CO2 sequestration relies not just on the gas exposure, but on the quality of the hydrated matrix prepared beforehand.
Summary Table:
| Feature | Curing Pre-treatment Phase | Carbonization Phase |
|---|---|---|
| Primary Goal | Hydraulic bonding & hydration | Chemical hardening & CO2 capture |
| Environment | 98% Relative Humidity (RH) | Lower Humidity (approx. 65% RH) |
| Key Outcome | Structural "skeleton" & pore network | Permanent CO2 sequestration |
| Risk of Failure | Micro-cracking & structural weakness | Inconsistent strength & poor gas uptake |
Precision curing is the foundation of high-performance building materials. Backed by expert R&D and manufacturing, KINTEK offers specialized curing solutions and laboratory systems—including Muffle, Tube, Rotary, Vacuum, and CVD furnaces—all customizable for your unique research needs. Ensure your magnesium slag mortar meets its full structural and sequestration potential. Contact our specialists today to optimize your lab process!
Visual Guide
References
- Gang Liu, Jianyun Wang. Effects of Hydraulic Materials on the Performance Evolution of Carbonated High-Volume Magnesium Slag Mortars. DOI: 10.3390/buildings15173062
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the role of a laboratory oven in the pretreatment of raw materials? Optimize EBC Powder Flowability
- What role does a laboratory circulating air drying oven play in the post-treatment of composite membranes? Master Stability
- What are the main types of sintering methods for metals, ceramics, and refractory intermetallic compounds powders? Optimize Your Material Processing
- What is the role of a rotary evaporator in the extraction of isopulegyl acetate? Protect Purity and Stability
- How do a fixed-bed reactor and an electric furnace ensure accuracy in evaluating hydrogen isotope catalytic oxidation?
- Why is the high-precision control of argon (Ar) and nitrogen (N2) flow ratios critical in CrSiN-Y coating fabrication?
- What is the purpose of adding aluminum in the vacuum distillation process for magnesium? Enhancing Process Stability and Purity
- Why is a reaction vessel with pressure control necessary for Ru nanoparticle synthesis? Achieve Precision Morphology



















