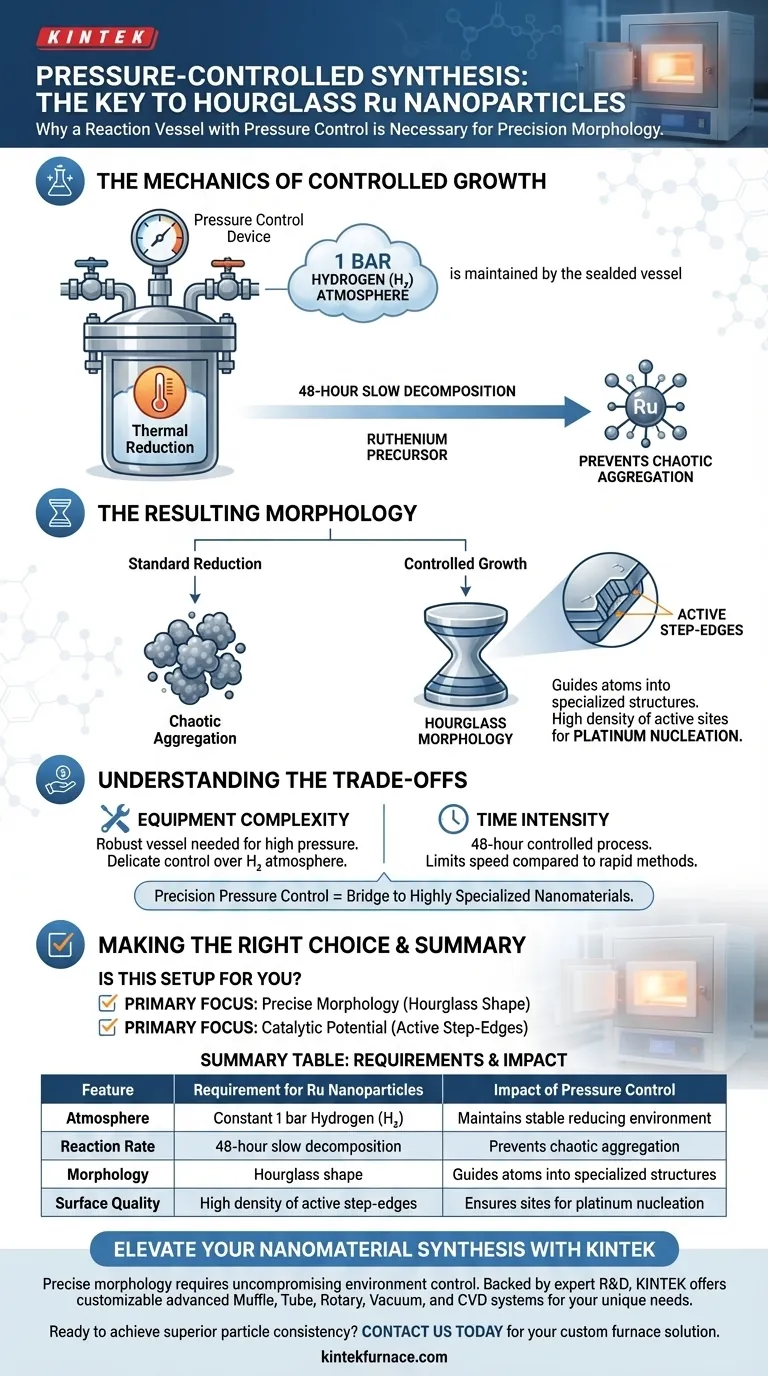
A reaction vessel equipped with a pressure control device is strictly necessary to maintain a stable reducing environment throughout the synthesis process. Specifically, it ensures a constant 1 bar hydrogen atmosphere is sustained during thermal reduction, allowing the ruthenium precursor to decompose at a highly controlled rate.
The pressure control device ensures the stability of the hydrogen atmosphere during a long, slow decomposition process. This controlled environment is the defining factor that forces ruthenium to grow into a unique hourglass morphology rich in active step-edges.

The Mechanics of Controlled Growth
Maintaining the Hydrogen Atmosphere
The synthesis of hourglass-shaped ruthenium nanoparticles relies on thermal reduction. This process requires a specific chemical environment: a 1 bar hydrogen atmosphere.
A standard vessel cannot guarantee the stability of this atmosphere under thermal stress. The pressure control device regulates the environment, ensuring the reducing agent (hydrogen) remains at the correct pressure throughout the reaction.
Enabling Slow Decomposition
This synthesis is not a rapid reaction; it is designed to be a slow, steady evolution. The ruthenium precursor undergoes decomposition over a period of 48 hours.
The pressure control device is essential for sustaining the necessary conditions over this extended timeframe. It prevents fluctuations that could accelerate decomposition too quickly or halt it entirely.
The Resulting Morphology
Achieving the Hourglass Shape
The precision provided by the pressure control device dictates the final physical form of the nanoparticle.
By enforcing a slow growth rate, the system prevents chaotic aggregation. Instead, it guides the ruthenium atoms to arrange themselves into a specialized hourglass morphology.
Creating Active Step-Edges
The ultimate goal of this morphology is functional, not aesthetic. The controlled growth results in a surface characterized by multiple step-edges.
These step-edges serve a critical purpose: they act as active sites. Specifically, they provide the necessary locations for the subsequent nucleation of platinum atoms in further applications.
Understanding the Trade-offs
Equipment Complexity
Using a pressure-controlled vessel increases the complexity of the experimental setup. The reaction vessel must be robust enough to withstand high pressures generated during the thermal process while maintaining delicate control over the hydrogen atmosphere.
Time Intensity
The reliance on a 48-hour controlled decomposition makes this a time-intensive synthesis method.
While this duration is necessary to achieve the step-edge morphology, it significantly limits the speed of production compared to rapid-reduction techniques that might yield less structured particles.
Making the Right Choice for Your Synthesis
To determine if this setup is required for your specific application, consider your end goals:
- If your primary focus is precise morphology: You must use pressure control to regulate the decomposition rate and achieve the hourglass shape.
- If your primary focus is catalytic potential: You must ensure the stability of the atmosphere for the full 48 hours to generate the step-edges required for platinum nucleation.
Precision pressure control is the bridge between a standard reduction reaction and the creation of highly specialized, functional nanomaterials.
Summary Table:
| Feature | Requirement for Ru Nanoparticles | Impact of Pressure Control |
|---|---|---|
| Atmosphere | Constant 1 bar Hydrogen ($H_2$) | Maintains stable reducing environment |
| Reaction Rate | 48-hour slow decomposition | Prevents chaotic aggregation |
| Morphology | Hourglass shape | Guides atoms into specialized structures |
| Surface Quality | High density of active step-edges | Ensures sites for platinum nucleation |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise morphology requires uncompromising environment control. Backed by expert R&D and manufacturing, KINTEK offers advanced Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique synthesis needs. Whether you are developing hourglass ruthenium particles or complex catalysts, our pressure-stabilized systems provide the reliability you need for a 48-hour controlled growth.
Ready to achieve superior particle consistency? Contact us today to discuss your custom furnace solution.
Visual Guide
References
- Qinyu Li, Richard D. Tilley. How the Arrangement of Platinum Atoms on Ruthenium Nanoparticles Improves Hydrogen Evolution Activity. DOI: 10.1002/adma.202509610
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
People Also Ask
- What experimental conditions do physical property measurement systems provide for TaAs2? Explore Cryogenic Transport
- Why is niobium foil wrapped around niobium cavity flanges? Protect Your UHV Seals During Heat Treatment
- Why are raw materials compacted into briquettes for vacuum carbothermal reduction? Optimize Your Magnesium Production
- Why are batch furnaces considered essential for certain applications? Achieve Precision and Flexibility in Heat Treatment
- How is the problem of surface oxidation and decarburization addressed in conventional heat treatment? Learn the Machining Allowance Method
- What is the function of PVA binder and high-pressure compression in SSBSN? Optimize Your Ceramic Green Body Preparation
- What are the advantages of using multi-stage laboratory sintering furnaces? Ensure Defect-Free Powder Metallurgy
- How does a precision temperature-controlled heating furnace enhance medium-entropy alloys? Achieve Optimal Hardness



















