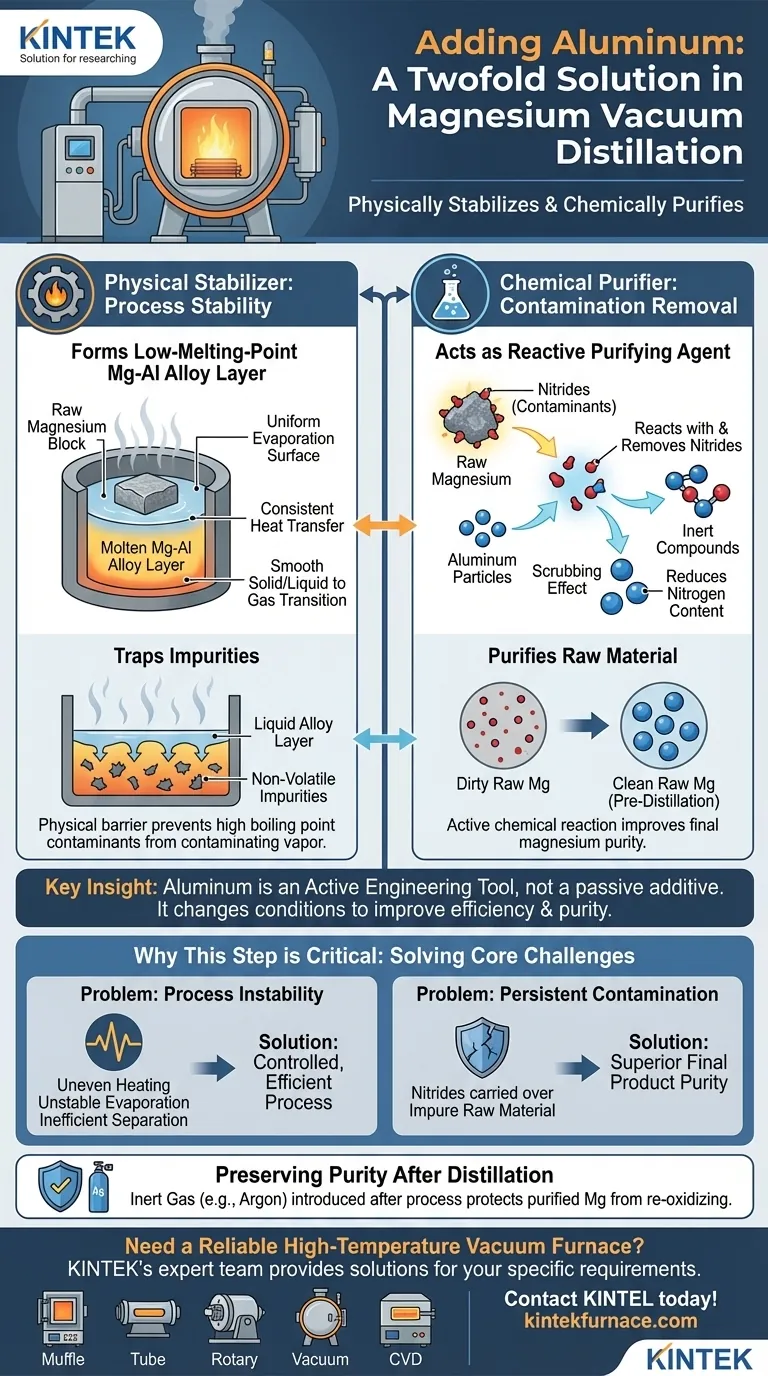
The purpose of adding aluminum during the vacuum distillation of magnesium is twofold: it physically stabilizes the evaporation process and chemically purifies the raw material. Aluminum forms a low-melting-point liquid alloy with magnesium, which creates a uniform surface for evaporation and traps impurities, while also reacting with and removing unwanted nitrides.
The key insight is that aluminum is not a passive additive but an active engineering tool. It fundamentally changes the physical and chemical conditions within the furnace to improve both the efficiency of the distillation and the purity of the final magnesium product.

The Dual Role of Aluminum in Magnesium Distillation
To understand the value of aluminum, you must recognize that it solves two distinct problems simultaneously. One is a physical challenge related to process stability, and the other is a chemical challenge related to contamination.
Creating a Stable Evaporation Interface
The first role of aluminum is to form a low-melting-point magnesium-aluminum alloy.
This liquid alloy spreads across the surface of the raw magnesium, creating a stable and uniform interface. Under vacuum, consistent evaporation is critical, and this liquid layer ensures even heat transfer and a smooth transition of magnesium from a solid or liquid to a gas.
Furthermore, this molten layer acts as a physical barrier, trapping other impurities that have higher boiling points and preventing them from contaminating the vapor stream.
Acting as a Chemical Purifying Agent
The second role of aluminum is to act as a reactive purifying agent.
Raw magnesium often contains contaminants, specifically nitrides, on its surface. These compounds can compromise the quality of the final product.
Aluminum actively reacts with these nitrides, effectively scrubbing them from the raw material. This chemical reaction reduces the final nitrogen content and significantly improves the overall purity of the distilled magnesium.
Why This Step is Critical: Solving Core Challenges
Adding aluminum isn't just an optimization; it addresses fundamental challenges inherent to the vacuum distillation process, ensuring a more reliable and higher-quality outcome.
The Problem of Process Instability
Vacuum distillation separates materials based on their different boiling points at low pressure. Any inconsistency in the raw material's surface can lead to uneven heating, unstable evaporation rates, and an inefficient separation.
The liquid Mg-Al alloy layer directly solves this by creating a predictable, homogenous surface, turning an unstable process into a controlled and efficient one.
The Persistent Threat of Contamination
Achieving high purity is the entire goal of distillation. However, simply boiling the magnesium off is not enough if certain contaminants can be carried over into the vapor or if the raw material itself is not properly prepared.
By chemically removing nitrides before the magnesium even evaporates, the aluminum addition ensures that the purification process starts with cleaner source material, leading to a superior final product.
Preserving Purity After Distillation
The focus on purity extends beyond the distillation itself. While not related to aluminum, it's important to note that an inert gas like argon is introduced after the process is complete.
This step protects the hot, highly reactive, and newly purified magnesium from re-oxidizing when it comes into contact with any residual oxygen, preserving the purity that was just achieved.
Making the Right Choice for Your Goal
The use of aluminum is a deliberate decision aimed at specific process improvements. Understanding its functions allows you to optimize for your primary objective.
- If your primary focus is process efficiency and stability: The key is using aluminum to form a liquid alloy layer, which guarantees uniform evaporation and physically traps non-volatile impurities.
- If your primary focus is maximizing product purity: The crucial function is aluminum's chemical reactivity, which actively removes nitride contaminants from the raw magnesium before distillation begins.
Ultimately, adding aluminum is a critical step that transforms magnesium distillation from a simple separation into a highly controlled and effective purification process.
Summary Table:
| Role of Aluminum | Key Function | Benefit |
|---|---|---|
| Physical Stabilizer | Forms a low-melting-point Mg-Al alloy layer | Creates a uniform evaporation surface, traps impurities |
| Chemical Purifier | Reacts with and removes nitride contaminants | Significantly increases the final magnesium purity |
| Overall Impact | Solves core challenges of instability and contamination | Enables a highly controlled and efficient purification process |
Need a Reliable High-Temperature Vacuum Furnace for Your Distillation or Purification Processes?
Just as aluminum is a critical tool for purifying magnesium, the right furnace is the foundation of your entire thermal process. Achieving high-purity results requires precise temperature control and a stable vacuum environment.
KINTEK's expert R&D and manufacturing team provides the thermal solutions you can depend on. We offer a range of high-temperature furnaces, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your unique application needs—whether you are refining metals, developing new materials, or conducting critical research.
Let us help you build a more efficient and reliable process. Contact KINTEL today to discuss your specific requirements and how our expertise can benefit your project.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the function of a vacuum sintering furnace in the SAGBD process? Optimize Magnetic Coercivity and Performance
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity
- What is the mechanism of a vacuum sintering furnace for AlCoCrFeNi2.1 + Y2O3? Optimize Your High-Entropy Alloy Processing
- Why is a high-vacuum environment necessary in copper slag impoverishment? Maximize Your Matte Separation Efficiency
- Why must sintering equipment maintain a high vacuum for high-entropy carbides? Ensure Phase Purity and Peak Density



















