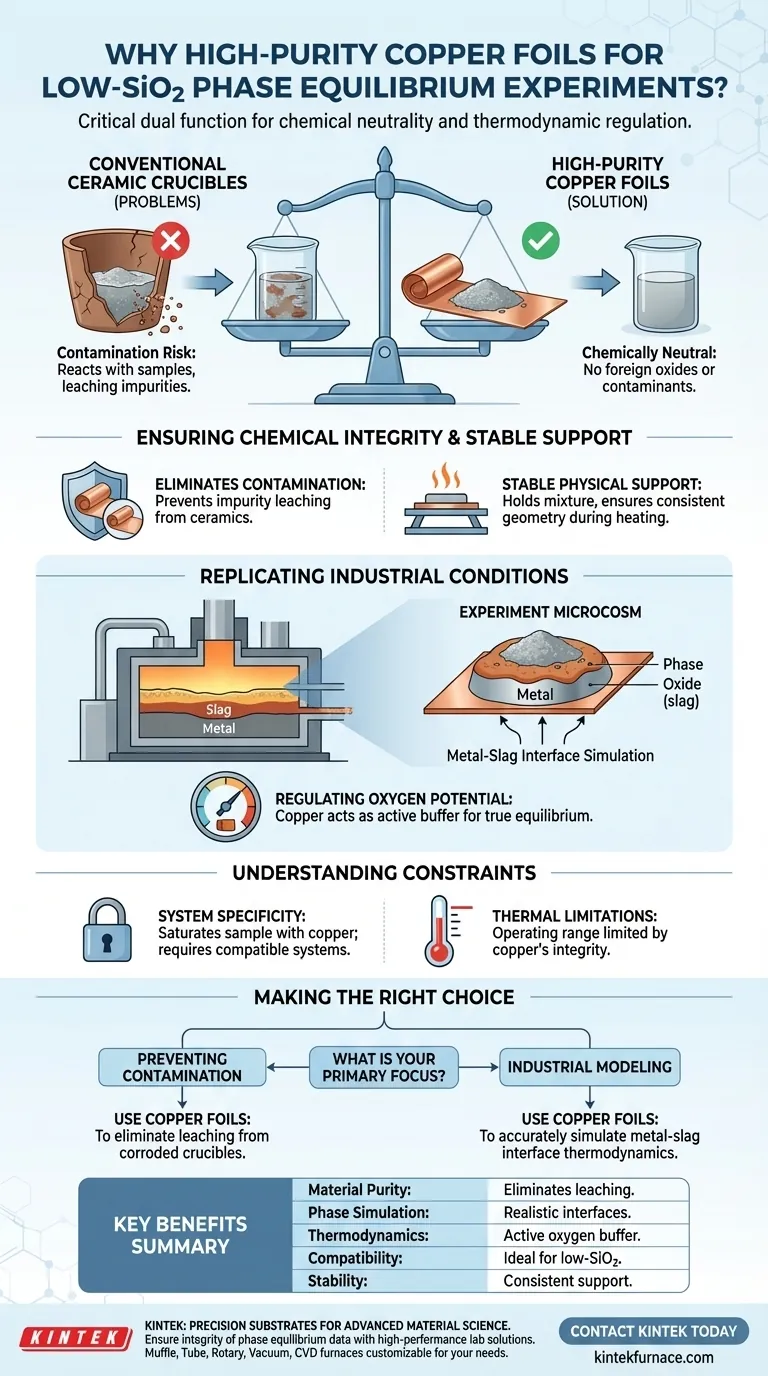
High-purity copper foils serve a critical dual function in phase equilibrium experiments: they provide a chemically neutral physical support and actively assist in regulating the thermodynamic environment. Specifically for low-silica mixtures equilibrated with cuprite (Cu2O) and senarmontite (Sb2O3), these foils enable researchers to replicate industrial conditions where metal and slag phases coexist, while eliminating the contamination risks associated with standard ceramic containers.
Core Takeaway Using copper foil substrates allows for precise control of oxygen potential and creates a realistic metal-slag interface without the chemical interference typical of ceramic crucibles. This setup is essential for obtaining accurate equilibrium data in low-silica systems.

Ensuring Chemical Integrity
Eliminating Ceramic Contamination
Conventional ceramic containers are prone to reacting with samples, particularly those with low silica content. This interaction can leach impurities into the mixture, compromising the data.
High-purity copper foils avoid this issue entirely. They provide a support structure that does not introduce foreign oxides or chemical contaminants into the melt.
Providing Stable Physical Support
The foil acts as a reliable mechanical substrate for the sample. It holds the mixture in place throughout the heating and equilibration process.
This stability ensures that the physical geometry of the sample remains consistent, which is necessary for accurate analysis.
Replicating Industrial Conditions
Simulating Phase Coexistence
Real-world industrial smelting and refining processes often involve the direct contact of metal phases and slag phases.
By using a copper substrate, researchers create a microcosm of this environment. The foil represents the bulk metal phase, allowing for the study of the interface and equilibrium between the metal and the oxide (slag) mixture.
Regulating Oxygen Potential
The copper foil is not merely a passive holder; it plays an active role in the system's thermodynamics.
It assists in buffering the oxygen potential of the system. This allows the sample to reach a true state of equilibrium under specific conditions of reduction or oxidation, consistent with the chemistry of cuprite and senarmontite interactions.
Understanding the Constraints
System Specificity
This method is highly specific to systems where copper is a compatible component.
Because the foil is in direct contact with the sample, the system effectively becomes saturated with copper. This technique is appropriate only when the experiment aims to study equilibrium in the presence of metallic copper.
Thermal Limitations
While not explicitly detailed in the reference, the use of copper foil naturally limits the operating temperature range.
The experiment must be conducted at temperatures where the copper foil maintains enough structural integrity to support the sample, or within the specific phase regions where the metal-slag equilibrium is being investigated.
Making the Right Choice for Your Experiment
To determine if copper foil substrates are the correct approach for your phase equilibrium study, consider the following specific goals:
- If your primary focus is preventing contamination: Use copper foils to eliminate the leaching of impurities often caused by the corrosion of ceramic crucibles in low-silica melts.
- If your primary focus is industrial modeling: Use copper foils to accurately simulate the thermodynamic interactions at the interface where metal phases and slag phases coexist.
By matching the substrate material to the specific chemical and physical requirements of the mixture, you ensure the integrity and relevance of your experimental data.
Summary Table:
| Feature | Benefit in Phase Equilibrium Experiments |
|---|---|
| Material Purity | Eliminates leaching and chemical contamination common in ceramic crucibles. |
| Phase Simulation | Replicates industrial metal-slag interfaces for realistic smelting data. |
| Thermodynamics | Acts as an active buffer to regulate oxygen potential and achieve equilibrium. |
| Compatibility | Ideal for low-SiO2 systems involving cuprite (Cu2O) and senarmontite (Sb2O3). |
| Stability | Provides consistent mechanical support throughout the high-temp heating process. |
Precision Substrates for Advanced Material Science
Ensure the integrity of your phase equilibrium data with high-performance laboratory solutions. KINTEK provides the specialized equipment needed to maintain precise thermodynamic conditions, backed by our expert R&D and world-class manufacturing.
Whether you require Muffle, Tube, Rotary, Vacuum, or CVD systems, our laboratory high-temp furnaces are fully customizable to meet the unique needs of your smelting simulations and low-silica research.
Ready to elevate your experimental accuracy? Contact KINTEK today to discuss your custom furnace needs.
Visual Guide
References
- Hamed Abdeyazdan, Evgueni Jak. Phase equilibria in the CuO <sub>0.5</sub> –SbO <sub>1.5</sub> –SiO <sub>2</sub> system. DOI: 10.1111/jace.70123
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Magnesium Extraction and Purification Condensing Tube Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What function does an electric blast drying oven serve in fluorite tailings activation? Ensure Process Precision
- What role does a microwave chemical reactor play in the synthesis of carbon xerogels? Precision Control & Efficiency
- What are the complexities and maintenance requirements of continuous furnaces? Optimize High-Volume Production with Expert Insights
- How does a precision temperature-controlled heating furnace enhance medium-entropy alloys? Achieve Optimal Hardness
- What experimental conditions do physical property measurement systems provide for TaAs2? Explore Cryogenic Transport
- What is the role of high-purity argon gas in ultrafine magnesium powder production? Control Particle Size & Purity
- What role do high-precision laboratory ovens play in assessing the energy potential of MSW? Enhancing Biomass Accuracy
- Why is an auxiliary gas supply device required for oil sludge pyrolysis? Ensure Stable Thermal Balance







