In short, high-purity argon gas is the primary control medium in the evaporation-condensation method for producing ultrafine magnesium powder. It provides an inert atmosphere and, most critically, its pressure directly determines the final size of the magnesium particles by governing how they form and grow.
The core principle to understand is that argon pressure is the main lever you can pull to tune the particle size. Higher argon pressure creates a denser environment, leading to more atomic collisions and ultimately larger magnesium powder particles.

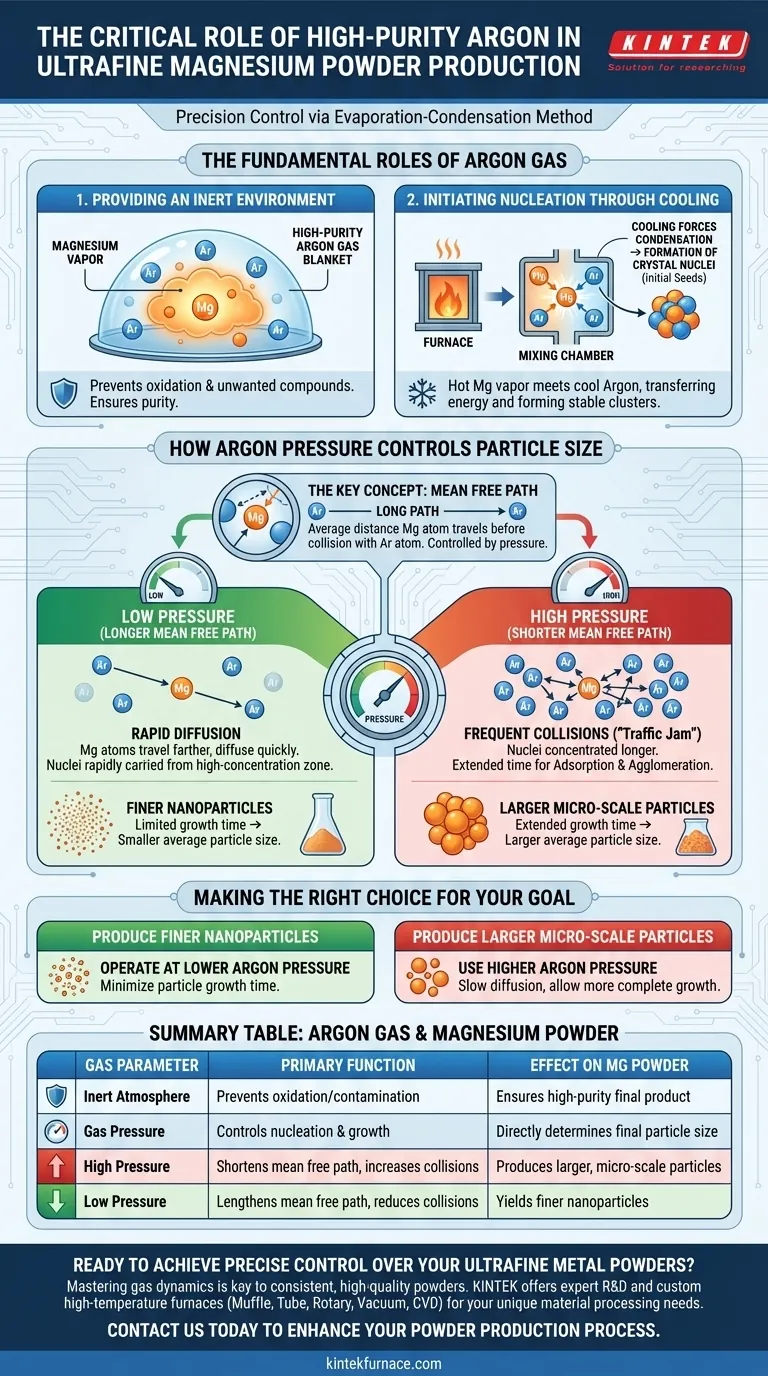
The Fundamental Roles of Argon Gas
To fully grasp the process, it's essential to break down the two critical functions argon performs: creating an inert environment and initiating the formation of powder particles.
Providing an Inert Environment
Magnesium is a highly reactive metal, especially in its vapor state at high temperatures. High-purity argon is chemically inert, meaning it won't react with the magnesium.
This creates a protective blanket, preventing the magnesium vapor from oxidizing or forming unwanted compounds, which ensures the purity of the final magnesium powder.
Initiating Nucleation Through Cooling
The process begins by heating solid magnesium until it evaporates into a hot vapor. This vapor is then introduced into a chamber filled with cooler, high-purity argon gas.
When the hot magnesium atoms collide with the cooler argon atoms, they transfer energy and rapidly cool down. This cooling forces the magnesium atoms to condense and form tiny, stable clusters known as crystal nuclei, the initial seeds of the final powder particles.
How Argon Pressure Controls Particle Size
The most powerful function of argon in this process is its role in controlling particle growth. The key to this control is a concept called the mean free path, which is directly manipulated by the gas pressure.
The Concept of Mean Free Path
The mean free path is the average distance a magnesium vapor atom can travel before it collides with an argon atom.
The pressure of the argon gas determines this distance. Lower pressure means fewer argon atoms and a longer mean free path, while higher pressure means more argon atoms and a much shorter mean free path.
The Effect of High Pressure
When the argon pressure is high, the mean free path for magnesium atoms is very short. This causes frequent collisions, creating greater resistance to the diffusion of the magnesium vapor.
This "traffic jam" effect keeps the newly formed crystal nuclei concentrated in a small area for a longer period. This extended time allows them to grow larger by attracting more magnesium atoms (adsorption) and sticking to other nuclei (agglomeration), resulting in a larger average particle size.
The Effect of Low Pressure
Conversely, at low argon pressure, the mean free path is long. Magnesium atoms travel farther between collisions and the vapor diffuses much more quickly.
The crystal nuclei are rapidly carried away from the high-concentration zone. This gives them very little time to grow, effectively "freezing" them at a very small size. This results in a much finer powder with a smaller average particle size.
Understanding the Key Trade-off
While argon pressure is a powerful tool, it's essential to understand the direct relationship it creates and the importance of process purity.
The Pressure-to-Size Relationship
The primary trade-off is simple: control over particle size. There is a direct and predictable correlation between the pressure you set and the powder you produce.
Failing to precisely control the argon pressure will lead to inconsistent batch-to-batch results, with variations in particle size distribution that can affect the powder's performance in its final application.
The Purity Imperative
The term "high-purity" is not incidental. Any contaminants in the argon gas, such as oxygen or water vapor, can react with the magnesium.
This contamination can introduce impurities into the final powder, compromising its chemical properties and performance. Maintaining the purity of the inert gas is therefore as critical as controlling its pressure.
Making the Right Choice for Your Goal
You can leverage this knowledge to precisely engineer the magnesium powder for a specific application by adjusting the argon gas pressure.
- If your primary focus is producing the finest possible nanoparticles: Operate at a lower argon gas pressure to minimize particle growth time.
- If your primary focus is producing larger, micro-scale particles: Use a higher argon gas pressure to slow vapor diffusion and allow for more complete particle growth.
Ultimately, mastering the pressure of the argon gas gives you direct and repeatable control over the physical characteristics of your final product.
Summary Table:
| Argon Gas Parameter | Primary Function | Effect on Magnesium Powder |
|---|---|---|
| Inert Atmosphere | Prevents oxidation and contamination | Ensures high-purity final product |
| Gas Pressure | Controls particle nucleation and growth | Directly determines final particle size |
| High Pressure | Shortens mean free path, increases collisions | Produces larger, micro-scale particles |
| Low Pressure | Lengthens mean free path, reduces collisions | Yields finer nanoparticles |
Ready to Achieve Precise Control Over Your Ultrafine Metal Powders?
Mastering gas dynamics is key to producing consistent, high-quality powders. At KINTEK, we understand the critical role of process parameters like argon pressure in achieving your desired particle size and purity.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temperature furnaces, all customizable for your unique material processing needs. Whether you're developing advanced materials or optimizing production, our solutions provide the precise environment control you require.
Contact us today using the form below to discuss how our equipment can enhance your powder production process and deliver the results you need.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing



















