The definitive answer is that the primary inert gases used in vacuum furnaces are argon (Ar) and nitrogen (N₂). They are introduced into the furnace chamber after a vacuum has been established to create a controlled, non-reactive atmosphere, which is critical for protecting materials during high-temperature processing.
The selection of an inert gas is not merely a background detail; it is a critical process variable. While both argon and nitrogen prevent destructive oxidation, the choice between them hinges on a crucial balance between the chemical reactivity of the material being processed, the required purity of the final product, and overall operational cost.

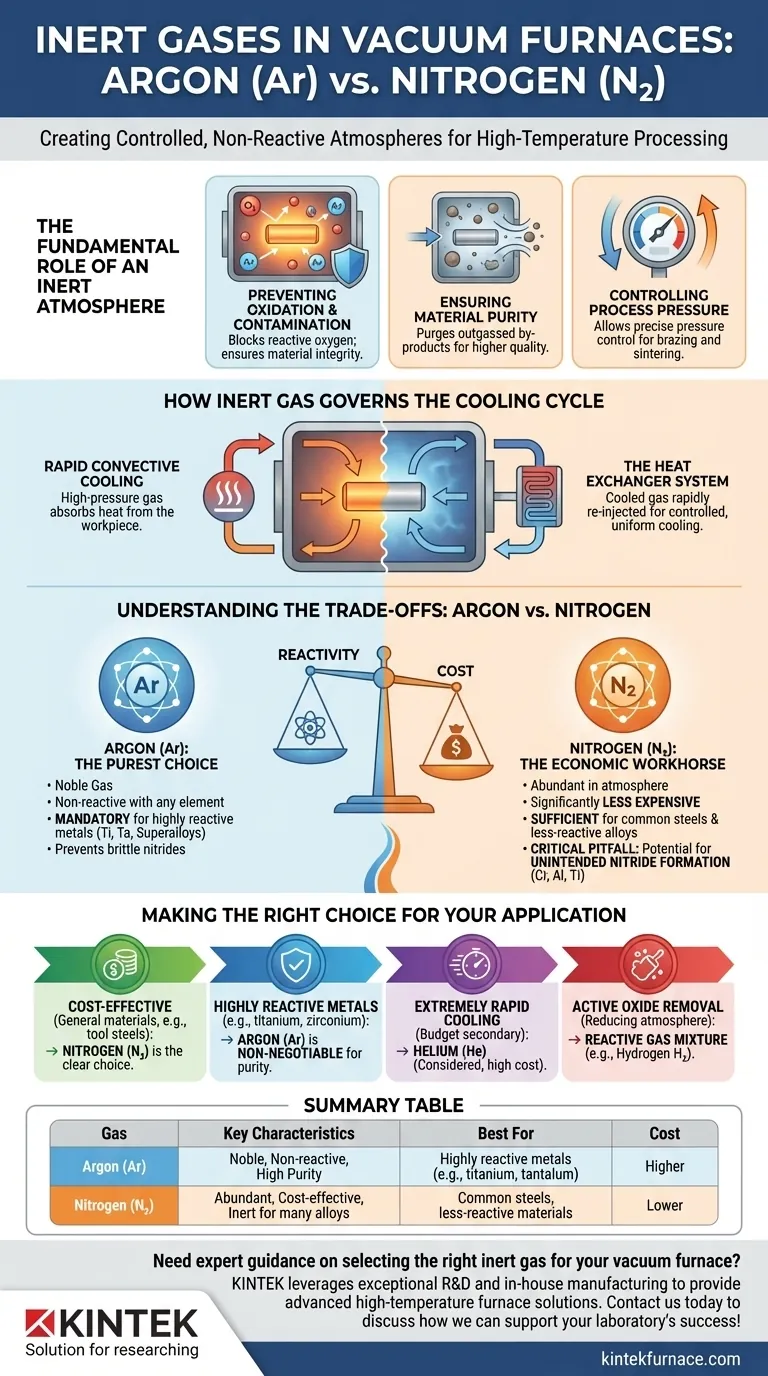
The Fundamental Role of an Inert Atmosphere
A vacuum furnace first removes the reactive gases from the chamber, primarily oxygen. However, a perfect vacuum is often impractical or undesirable. Backfilling with an inert gas serves several essential functions.
Preventing Oxidation and Contamination
At the extreme temperatures inside a furnace, most materials become highly reactive. Any residual oxygen would immediately cause oxidation, compromising the material's integrity.
An inert gas provides a safe, non-reactive environment. This prevents unwanted chemical reactions, ensuring materials like high-strength alloys or pure metals maintain their specific characteristics.
Ensuring Material Purity
The process of heating can cause materials to release by-products, a phenomenon known as outgassing.
An inert atmosphere helps purge these released contaminants from the heating zone. This continuous removal of by-products during the process results in a final product with significantly higher purity.
Controlling Process Pressure
Many advanced thermal processes require a specific partial pressure, not a hard vacuum. This is impossible to achieve without introducing a gas.
By backfilling with argon or nitrogen, operators can precisely control the chamber pressure. This pressure control is vital for processes like brazing or sintering, where it can influence material flow and density.
How Inert Gas Governs the Cooling Cycle
Beyond creating a passive environment, inert gas plays an active and critical role in the cooling phase of a furnace cycle, often called quenching.
Enabling Rapid Convective Cooling
A vacuum is an excellent insulator, which means it also prevents heat from escaping. To cool parts quickly, heat must be actively removed.
Introducing a high-pressure inert gas into the hot zone allows for convective cooling. The gas absorbs heat from the hot workpiece and is then circulated away.
The Heat Exchanger System
This process is managed by a closed-loop system. The hot gas is pulled from the furnace chamber and forced through a heat exchanger, which cools it back to room temperature.
This cooled, dense gas is then reinjected into the furnace at high velocity, rapidly and uniformly drawing heat from the product. This controlled, quick cooling is essential for achieving specific metallurgical properties and hardness in metals.
Understanding the Trade-offs: Argon vs. Nitrogen
The choice between the two primary gases is a critical engineering decision based on material science and economics.
Argon (Ar): The Purest Choice
Argon is a noble gas, meaning it is almost completely non-reactive with any other element at any temperature.
This makes it the mandatory choice for processing highly reactive metals such as titanium, tantalum, and certain nickel-based superalloys. Using nitrogen with these materials would result in the formation of brittle nitrides, ruining the component.
Nitrogen (N₂): The Economic Workhorse
Nitrogen is far more abundant in the atmosphere than argon, making it significantly less expensive.
For the vast majority of heat-treating applications involving common steels and other less-reactive alloys, nitrogen provides a perfectly sufficient inert atmosphere. Its cost-effectiveness makes it the default choice when possible.
The Critical Pitfall: Unintended Nitride Formation
The primary trade-off with nitrogen is its potential to react with certain elements at high temperatures. Elements like chromium, aluminum, and titanium can bond with nitrogen to form nitrides.
While sometimes this is a desirable outcome (in a surface-hardening process called nitriding), it is often an unintended and detrimental form of contamination that alters material properties. A thorough material compatibility check is essential before using nitrogen.
Making the Right Choice for Your Application
Your material, process requirements, and budget will dictate the optimal gas selection.
- If your primary focus is cost-effectiveness for general-purpose materials (e.g., tool steels): Nitrogen is the clear choice, offering sufficient inertness at a much lower cost.
- If your primary focus is processing highly reactive metals (e.g., titanium, zirconium): Argon is non-negotiable to prevent nitride formation and ensure maximum material purity.
- If your primary focus is extremely rapid cooling and budget is secondary: Helium, with its superior thermal conductivity, can be considered, but its high cost and difficulty in containment are major drawbacks.
- If your primary focus is active oxide removal, not inertness: A reactive gas mixture containing hydrogen is used, which actively strips oxygen from surfaces in a reducing atmosphere.
By understanding these core principles, you can select an atmosphere that actively contributes to the quality and integrity of your final product.
Summary Table:
| Gas | Key Characteristics | Best For | Cost |
|---|---|---|---|
| Argon (Ar) | Noble gas, non-reactive, high purity | Highly reactive metals (e.g., titanium, tantalum) | Higher |
| Nitrogen (N₂) | Abundant, cost-effective, inert for many alloys | Common steels, less-reactive materials | Lower |
Need expert guidance on selecting the right inert gas for your vacuum furnace? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements, helping you achieve optimal material purity and process efficiency. Contact us today to discuss how we can support your laboratory's success!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments



















