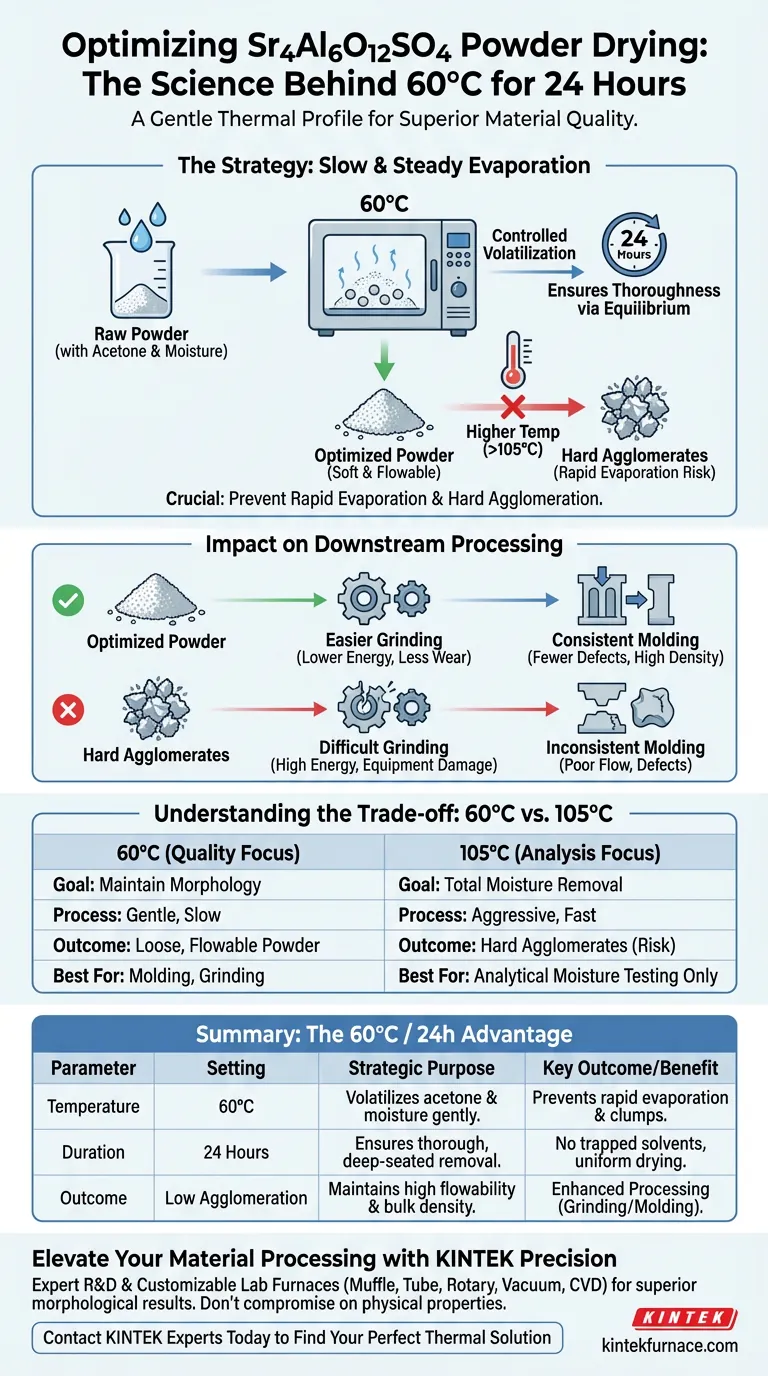
The primary purpose of the 60°C, 24-hour drying cycle is to thoroughly remove acetone solvents and adsorbed moisture without damaging the physical structure of the powder. This specific thermal profile is designed to be a gentle, low-heat treatment rather than an aggressive drying phase.
By prioritizing a slow evaporation rate over speed, this process prevents the formation of hard agglomerates. This ensures the Sr4Al6O12SO4 powder retains the high flowability and bulk density necessary for successful downstream processing.

The Strategy Behind Low-Temperature Drying
To understand why this specific protocol is used, one must look beyond simple moisture removal and consider the mechanics of particle formation.
Targeting Volatile Components
The preparation phase of Sr4Al6O12SO4 involves mixing solvents, specifically acetone, along with incidental moisture.
The 60°C setting is sufficient to volatilize these components effectively over time.
Preventing Structural Defects
The critical variable here is the rate of evaporation.
If the temperature were raised significantly higher to speed up the process, the moisture and solvents would evaporate rapidly.
This rapid exit of volatiles often causes particles to pull together tightly, resulting in hard agglomeration.
Ensuring Thoroughness
The extended duration of 24 hours compensates for the lower temperature.
This creates a steady, equilibrium-driven drying process that removes deep-seated solvent traces that a shorter, hotter blast might trap inside a hardened outer shell.
Impact on Downstream Processing
The quality of the drying phase directly dictates the success of the subsequent manufacturing steps.
Optimizing for Grinding
Because the low-heat treatment prevents hard clumps from forming, the resulting material is softer and more uniform.
This makes the powder significantly easier to grind, reducing energy consumption and equipment wear.
Facilitating Molding
Flowability and bulk density are two properties preserved by this gentle drying method.
When the powder flows freely and packs densely, it fills molds more consistently, leading to fewer defects in the final formed part.
Understanding the Trade-offs
It is common in other industries to use higher temperatures for drying, but those rules do not apply here.
Comparing 60°C vs. 105°C
Standard drying protocols—such as those used for fuel samples—often utilize ovens set to 105°C.
In those contexts, the goal is the absolute removal of physical moisture to prevent endothermic reactions during high-temperature combustion experiments.
The Risk of High Heat
However, applying that 105°C standard to Sr4Al6O12SO4 would likely be detrimental.
While it would dry the powder faster, the intense heat would trigger the rapid evaporation mentioned earlier, sacrificing the morphological quality of the powder for the sake of speed.
Making the Right Choice for Your Goal
When determining your drying parameters, you must align the temperature with your material requirements.
- If your primary focus is Powder Quality: Stick to 60°C for 24 hours to ensure the removal of acetone while maintaining a loose, flowable structure for molding.
- If your primary focus is Analytical Moisture Measurement: Use 105°C (as seen in fuel analysis) only if you are testing for total moisture content and do not intend to use the sample for further molding or shaping.
Ultimately, the 60°C cycle is an investment in the material's physical handling properties, ensuring the powder remains workable for the rest of the production line.
Summary Table:
| Parameter | Setting | Strategic Purpose |
|---|---|---|
| Temperature | 60°C | Volatilizes acetone & moisture without triggering rapid evaporation. |
| Duration | 24 Hours | Ensures thorough removal of deep-seated solvents via equilibrium. |
| Key Outcome | Low Agglomeration | Prevents hard clumps, maintaining high flowability and bulk density. |
| Benefit | Enhanced Processing | Easier grinding and more consistent mold filling for final parts. |
Elevate Your Material Processing with KINTEK
Precision thermal control is the difference between a high-quality powder and a failed batch. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp furnaces—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all of which are fully customizable to meet your unique drying and sintering requirements.
Whether you are processing Sr4Al6O12SO4 or developing new advanced ceramics, our equipment ensures the stable, uniform heat distribution necessary for superior morphological results. Don't compromise on your material's physical properties.
Contact KINTEK Experts Today to Find Your Perfect Thermal Solution
Visual Guide
References
- José A. Rodríguez‐García, Enrique Rocha‐Rangel. Chemical Interaction between the Sr4Al6O12SO4 Ceramic Substrate and Al–Si Alloys. DOI: 10.3390/eng5010025
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why Use a Vacuum Oven for Cu-Cu2O/g-C3N4 Catalysts? Preserve Purity and Structural Integrity
- What are the three types of dental ceramics? A Guide to Material Selection
- How do precision electric drying ovens control the precipitation of strengthening phases in recycled aluminum alloys?
- What is the purpose of pre-drying SiO2 raw materials at 400 degrees Celsius? Ensure Precise Stoichiometric Synthesis
- What is the primary purpose of operating a laboratory oven at 383 K for 24 hours? Precision Drying for Carbon Prep
- Why is the purity of oxide precursors critical for ZnO-doped CuO? Ensure High Photocatalytic Performance
- What role does pack media play in the solid-state powder boriding process? Enhance Metal Hardness at High Temperatures
- Why are User-Defined Functions (UDFs) necessary for modeling complex combustion? Unlock Precision in Furnace Simulation



















