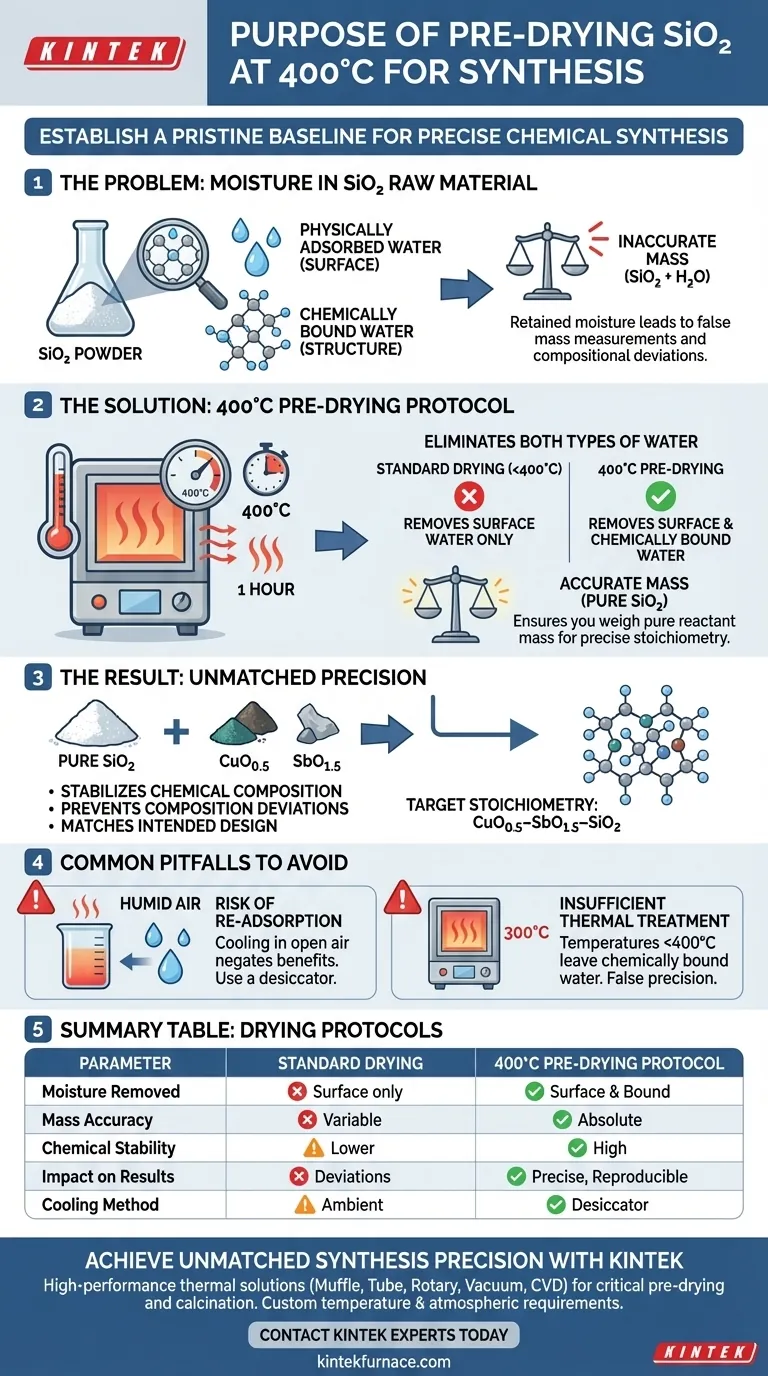
The primary purpose of pre-drying SiO2 (silicon dioxide) at 400°C is to establish a pristine baseline for your chemical synthesis by removing all traces of moisture. This specific thermal treatment is calibrated to eliminate both physically adsorbed water found on the surface and chemically bound water integrated into the powder's structure.
Precision in solid-state synthesis relies entirely on the accuracy of your starting masses. Pre-drying ensures that when you weigh your SiO2, you are measuring the mass of the reactant itself, not the weight of the water contaminants clinging to it.

The Critical Role of Moisture Removal
Eliminating Two Types of Water
Standard drying at lower temperatures often removes only surface moisture. However, the protocol of heating to 400°C for one hour is necessary to drive off both physically adsorbed water and chemically bound water.
Ensuring Pure Reactant Mass
If water remains in the powder, it contributes to the total weight measured on the balance. This means the actual amount of active SiO2 in your mix would be lower than calculated.
Stabilizing the Chemical Composition
By removing these volatile components, you ensure the raw material is chemically stable. This guarantees that the powder introduced to the mixture is pure SiO2, preventing unknown variables from entering the reaction.
Stoichiometry and Experimental Accuracy
Preventing Composition Deviations
The success of synthesizing CuO0.5–SbO1.5–SiO2 depends on achieving specific mass proportions. Even a small percentage of retained moisture can skew these ratios significantly.
Matching the Intended Design
Pre-drying ensures the final synthesized mixture aligns exactly with the theoretical chemical composition. Without this step, the final stoichiometry would drift, potentially altering the physical or chemical properties of the resulting sample.
Common Pitfalls to Avoid
The Risk of Re-adsorption
A common error is drying the material properly but allowing it to cool in humid air. SiO2 can rapidly re-absorb moisture from the atmosphere, negating the benefits of the heating process.
Insufficient Thermal Treatment
Using temperatures significantly lower than 400°C may leave chemically bound water behind. This results in "false precision," where the researcher believes the sample is dry, but the mass calculation remains inaccurate.
Making the Right Choice for Your Synthesis
To ensure the integrity of your CuO0.5–SbO1.5–SiO2 samples, apply the following principles:
- If your primary focus is Compositional Accuracy: Strict adherence to the 400°C limit is required to remove chemically bound water that standard drying ovens miss.
- If your primary focus is Reproducibility: Treat the cooling phase as critical; transfer the hot powder immediately to a desiccator to prevent moisture from returning.
By standardizing the pre-drying process, you transform a variable raw material into a reliable constant for your research.
Summary Table:
| Parameter | Standard Drying | 400°C Pre-Drying Protocol |
|---|---|---|
| Moisture Removed | Surface/Physically adsorbed water only | Both physically adsorbed and chemically bound water |
| Mass Accuracy | Variable (water weight included) | Absolute (pure reactant mass) |
| Chemical Stability | Lower (volatile components remain) | High (stable baseline for reaction) |
| Impact on Results | Stoichiometric deviations | Precise, reproducible composition |
| Cooling Method | Ambient air (re-adsorption risk) | Desiccator recommended |
Achieve Unmatched Synthesis Precision with KINTEK
Don't let moisture compromise your experimental accuracy. Whether you are synthesizing CuO0.5–SbO1.5–SiO2 or advanced ceramic composites, KINTEK provides the high-performance thermal solutions you need.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific temperature and atmospheric requirements. Our laboratory high-temp furnaces ensure uniform heating and stable thermal profiles for critical pre-drying and calcination steps.
Ready to elevate your research consistency?
Contact KINTEK experts today to find the perfect furnace for your laboratory’s unique needs.
Visual Guide
References
- Hamed Abdeyazdan, Evgueni Jak. Phase equilibria in the CuO <sub>0.5</sub> –SbO <sub>1.5</sub> –SiO <sub>2</sub> system. DOI: 10.1111/jace.70123
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is High-Temperature Annealing Required for WS2 Gas Sensors? Stabilize Performance & Eliminate Drift
- What are the primary functions of a high-precision dilatometer in hot ductility? Optimize Steel Casting Precision
- Why is a forced air circulation oven required for Al-Cu-Mn alloy aging? Achieve Peak Hardness with Uniform Heat
- What are the main types of laboratory furnaces based on size? Find the Perfect Fit for Your Lab's Scale
- How does the Flash Heating (FH) process impact the growth of REBCO films? Master Rapid Thermal Ramp Requirements
- How do heating and stirring support chemical synthesis? Optimize Reaction Kinetics and Thermodynamics
- What is the primary function of a high-precision drop furnace? Master Flash Smelting Simulation Kinetics
- What is the function of a rotary high-pressure autoclave in the synthesis of SSZ-13 zeolites? | Enhance Crystallinity



















