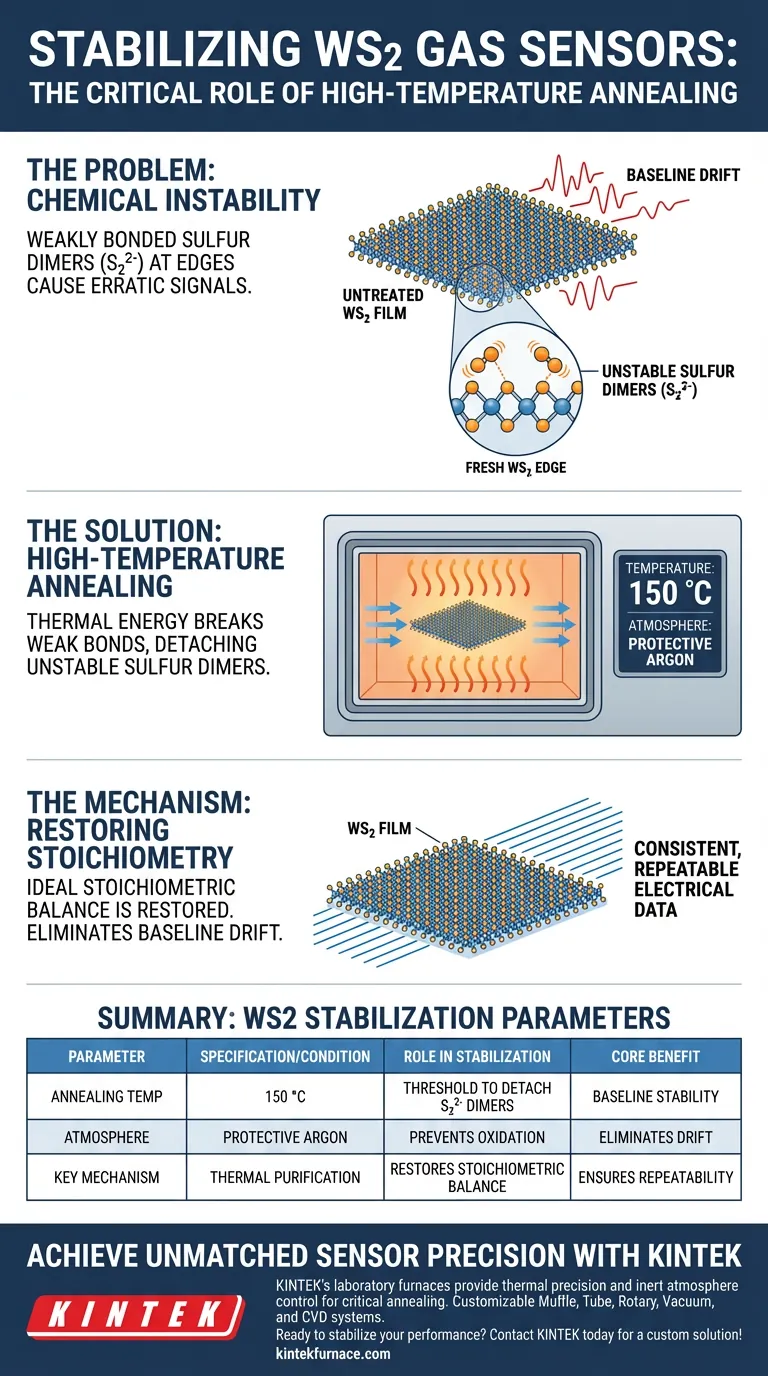
High-temperature annealing is the critical processing step required to eliminate chemical instabilities that plague untreated tungsten disulfide (WS2) sensors. By subjecting the sensing element to 150 °C under a protective argon atmosphere, you remove unstable sulfur groups from the material's edges, ensuring the device yields consistent, repeatable electrical data rather than erratic signals.
The annealing process physically strips away weakly bonded sulfur dimers ($S_2^{2-}$), restoring the material's ideal stoichiometric balance. This chemical purification is the specific mechanism that eliminates baseline drift, transforming a volatile thin film into a reliable sensor for room-temperature applications.
The Chemistry of Instability
The Problem with "Fresh" WS2 Edges
When tungsten disulfide thin films are fabricated, the edges of the material are rarely perfect.
They often harbor unstable chemical groups that attach loosely to the crystal structure.
Identifying the Culprit: Sulfur Dimers
The primary source of electrical noise in these sensors is the presence of weakly bonded sulfur dimers ($S_2^{2-}$).
These groups cling to the edges of the WS2 film but lack the strong covalent bonding of the core material.
Consequences for Performance
These unstable groups are electrically active in unpredictable ways.
They cause the sensor's baseline signal to drift, meaning the sensor reports a change in resistance even when no gas is present.
Without addressing this, the sensor suffers from poor repeatability, rendering it useless for precise measurement.
The Mechanism of Stabilization
Using Heat to Purify
The annealing process utilizes a high-temperature laboratory environment, specifically set to 150 °C.
This thermal energy is calibrated to be high enough to break the weak bonds of the unstable sulfur dimers, effectively detaching them from the film.
Protective Atmosphere
This process is strictly performed under an argon protective atmosphere.
Argon is an inert gas, which ensures that as the material heats up, the tungsten disulfide does not react with oxygen or moisture in the air.
Restoring Stoichiometry
By removing the excess sulfur dimers, the material is brought closer to its ideal stoichiometric state.
This creates a chemically stable surface where the electrical properties are defined by the WS2 crystal structure, not by edge defects.
Understanding the Process Constraints
The Necessity of Temperature Control
The target temperature of 150 °C is not arbitrary.
It represents the specific thermal threshold required to remove the unstable groups without degrading the underlying thin film.
The Cost of Stability
Achieving this stability requires specialized equipment to maintain the argon atmosphere.
This adds a layer of complexity compared to simple air annealing, but it is a necessary trade-off to prevent oxidation while removing the sulfur defects.
Optimizing Sensor Fabrication
To ensure your tungsten disulfide sensors perform reliably in the field, you must view annealing as a chemical correction step, not just a drying process.
- If your primary focus is baseline stability: You must ensure the annealing temperature reaches 150 °C to successfully detach weakly bonded sulfur dimers ($S_2^{2-}$).
- If your primary focus is repeatability: You must maintain a strict argon atmosphere to prevent surface contamination while the material’s stoichiometry is being restored.
By effectively removing edge defects, you convert a raw semiconductor material into a precision instrument capable of consistent room-temperature sensing.
Summary Table:
| Parameter | Specification/Condition | Role in WS2 Stabilization |
|---|---|---|
| Annealing Temp | 150 °C | Threshold to detach unstable sulfur dimers ($S_2^{2-}$) |
| Atmosphere | Protective Argon | Prevents oxidation and reaction with air/moisture |
| Key Mechanism | Thermal Purification | Restores stoichiometric balance at material edges |
| Core Benefit | Baseline Stability | Eliminates signal drift and ensures repeatability |
Achieve Unmatched Sensor Precision with KINTEK
Don't let signal drift compromise your research. KINTEK’s high-performance laboratory furnaces provide the thermal precision and inert atmosphere control essential for the critical annealing of WS2 and other 2D materials.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific gas-sensing fabrication needs.
Ready to stabilize your sensor performance? Contact KINTEK today for a custom solution!
Visual Guide
References
- Thin Films of Tungsten Disulfide Grown by Sulfurization of Sputtered Metal for Ultra-Low Detection of Nitrogen Dioxide Gas. DOI: 10.3390/nano15080594
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the specific function of hydrogen and helium in quartz glass melting? Optimize Your High-Temp Processes
- How do heating and stirring support chemical synthesis? Optimize Reaction Kinetics and Thermodynamics
- Why is a stainless steel high-pressure autoclave essential for starch hydrogenation? Unlock Peak Reaction Efficiency
- What are the advantages of using a customized multimode microwave reaction furnace? Boost Synthesis Speed by 90%
- What role does a high-power graphite resistance furnace play in SiC ceramic treatment? Achieve Perfect Crystallization
- Why is a vacuum drying oven required for the pretreatment of modified zeolite? Preserve Pore Integrity for CO2 Capture
- What is Joule Heating and how does it relate to induction heating? Master the Physics of Contactless Heating
- What are the functions of hot isostatic pressing (HIP) equipment? Achieve Peak Density in Powder Metallurgy



















