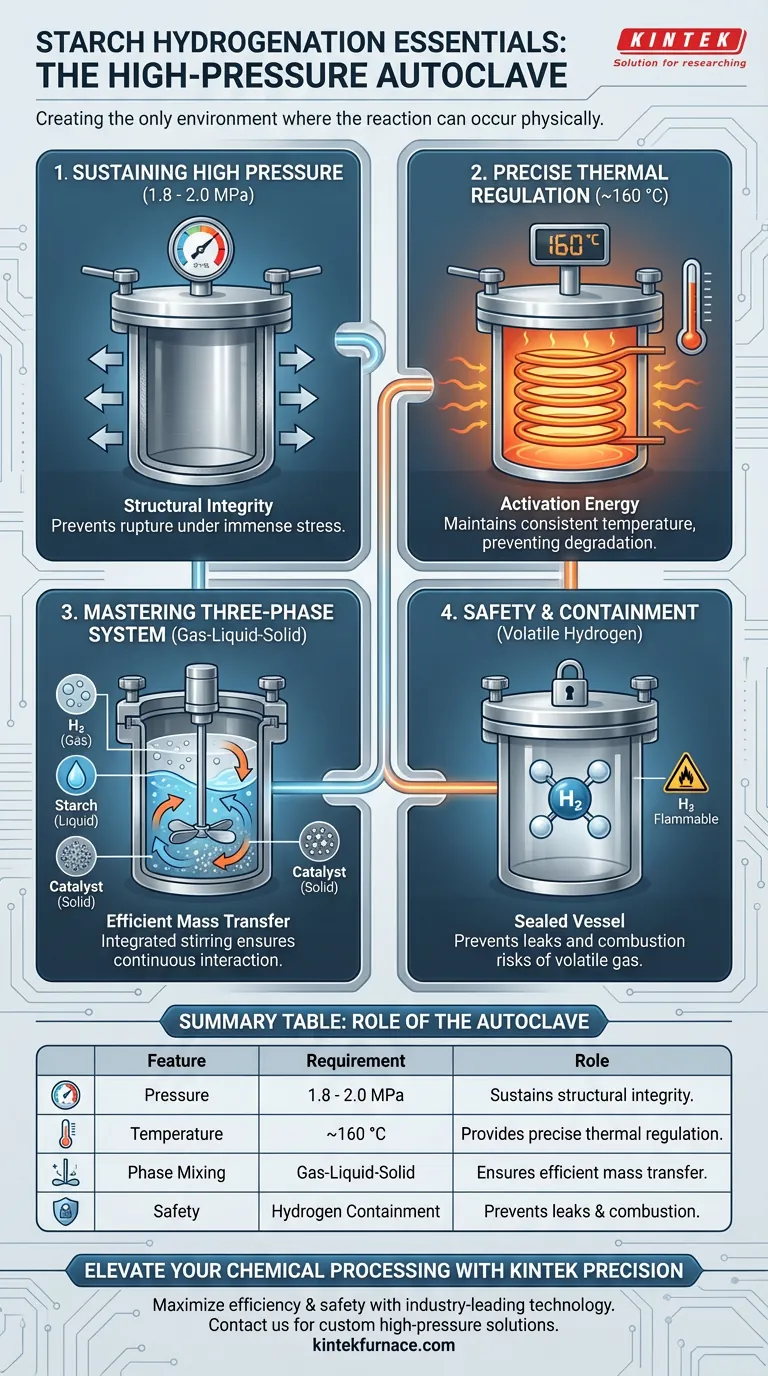
A stainless steel high-pressure autoclave is essential because it creates the only environment where starch hydrogenation can physically occur. It provides a sealed, robust vessel capable of sustaining pressures between 1.8 and 2.0 MPa and temperatures around 160 °C. Furthermore, it incorporates the mechanical agitation necessary to mix hydrogen gas, liquid starch, and solid catalysts safely.
Starch hydrogenation is a complex, multiphase reaction that demands aggressive environmental conditions. The autoclave serves as a pressurized containment system that forces hydrogen into solution while simultaneously managing the safety risks of high-pressure gas.

Creating the Required Reaction Environment
To convert starch into sugar alcohols efficiently, standard atmospheric conditions are insufficient. The autoclave bridges the gap between the reactants and the necessary activation energy.
Sustaining High Pressure
The primary barrier to this reaction is pressure. The process requires a steady internal pressure of 1.8 to 2.0 MPa.
Standard vessels would rupture or leak under this stress. The stainless steel construction ensures the structural integrity needed to hold this pressure safely for the duration of the reaction.
Precise Thermal Regulation
Pressure alone is not enough; thermal energy is required to drive the chemical conversion.
The vessel maintains a consistent temperature, typically 160 °C. The autoclave allows for precise thermal control, preventing temperature spikes that could degrade the starch or stall the reaction.
Mastering the Three-Phase System
The most difficult engineering challenge in starch hydrogenation is that the reactants exist in three different states: gas, liquid, and solid.
The Gas-Liquid-Solid Challenge
The reaction involves hydrogen (gas), the starch solution (liquid), and a catalyst (solid).
For the reaction to work, these three distinct phases must interact intimately. If they separate, the hydrogenation process stops immediately.
Enhancing Mass Transfer via Stirring
The autoclave solves the separation issue with an integrated stirring system.
This mechanism is critical for efficient mass transfer. It actively disperses the hydrogen gas bubbles throughout the liquid and keeps the solid catalyst suspended, ensuring all three components collide and react continuously.
Safety and Operational Considerations
While the autoclave is the correct tool for the job, using high-pressure equipment introduces specific operational demands.
Containing Volatile Hydrogen
Hydrogen is highly flammable and difficult to contain due to its small molecular size.
The "sealed reaction space" provided by the autoclave is a critical safety feature. It prevents hydrogen leaks, which could lead to combustion or explosion outside the vessel.
The Complexity of Maintenance
Operating at 2.0 MPa imposes significant stress on seals and mechanical parts.
Operators must recognize that the "stability" provided by the vessel relies on rigorous maintenance. If the stirring seals fail, the pressure integrity is compromised, risking both the batch quality and operator safety.
Optimizing Your Equipment Choice
When selecting or operating a high-pressure autoclave for this specific application, focus on the following parameters.
- If your primary focus is Reaction Speed: Prioritize the efficiency of the integrated stirring system to maximize the contact between hydrogen gas and the liquid starch.
- If your primary focus is Process Safety: Ensure the vessel is rated significantly higher than the operating max of 2.0 MPa and inspect the seal integrity for hydrogen containment.
The autoclave is not just a container; it is an active participant that forces the physics of the reaction to occur.
Summary Table:
| Feature | Requirement for Starch Hydrogenation | Role of the Autoclave |
|---|---|---|
| Pressure | 1.8 - 2.0 MPa | Sustains structural integrity under high stress |
| Temperature | Approximately 160 °C | Provides precise thermal regulation and activation energy |
| Phase Mixing | Gas-Liquid-Solid Interaction | Integrated stirring system ensures efficient mass transfer |
| Safety | Hydrogen Containment | Sealed vessel prevents leaks and combustion risks |
Elevate Your Chemical Processing with KINTEK Precision
Maximize the efficiency of your starch hydrogenation and high-pressure reactions with KINTEK’s industry-leading technology. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside fully customizable lab high-temperature furnaces designed to meet your unique specifications.
Whether you need to master three-phase mass transfer or ensure absolute safety in volatile environments, our engineering team is ready to deliver the robust solutions your lab requires.
Ready to optimize your reaction environment? Contact KINTEK today to discuss your custom equipment needs!
Visual Guide
References
- Shenghua Zhu, Jinghua Liang. Forming a Cu-Based Catalyst for Efficient Hydrogenation Conversion of Starch into Glucose. DOI: 10.3390/catal14020132
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Vacuum Heat Treat Sintering and Brazing Furnace
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
People Also Ask
- What is the purpose of applying a hexagonal Boron Nitride (h-BN) coating to graphite? Enhance Purity & Tool Longevity
- How does the required process atmosphere affect the decision to use separate or combined furnaces for debinding and sintering? Optimize Your MIM Process
- What are some examples of high-temperature industrial heating processes? Explore Key Applications and Benefits
- What is the primary function of glass matrices in HLW vitrification? Achieve Safe Radioactive Waste Immobilization
- What processes can continuous furnaces perform in a single step? Master Debinding and Sintering for High-Volume Production
- What is the core role of a high-pressure autoclave in the synthesis of LTA zeolites? Achieve Precise Crystal Growth
- Why is carbon dioxide utilized for the in-situ gasification regeneration of NiCuCe catalysts? Enhance Catalyst Longevity
- What is Joule Heating and how does it relate to induction heating? Master the Physics of Contactless Heating



















