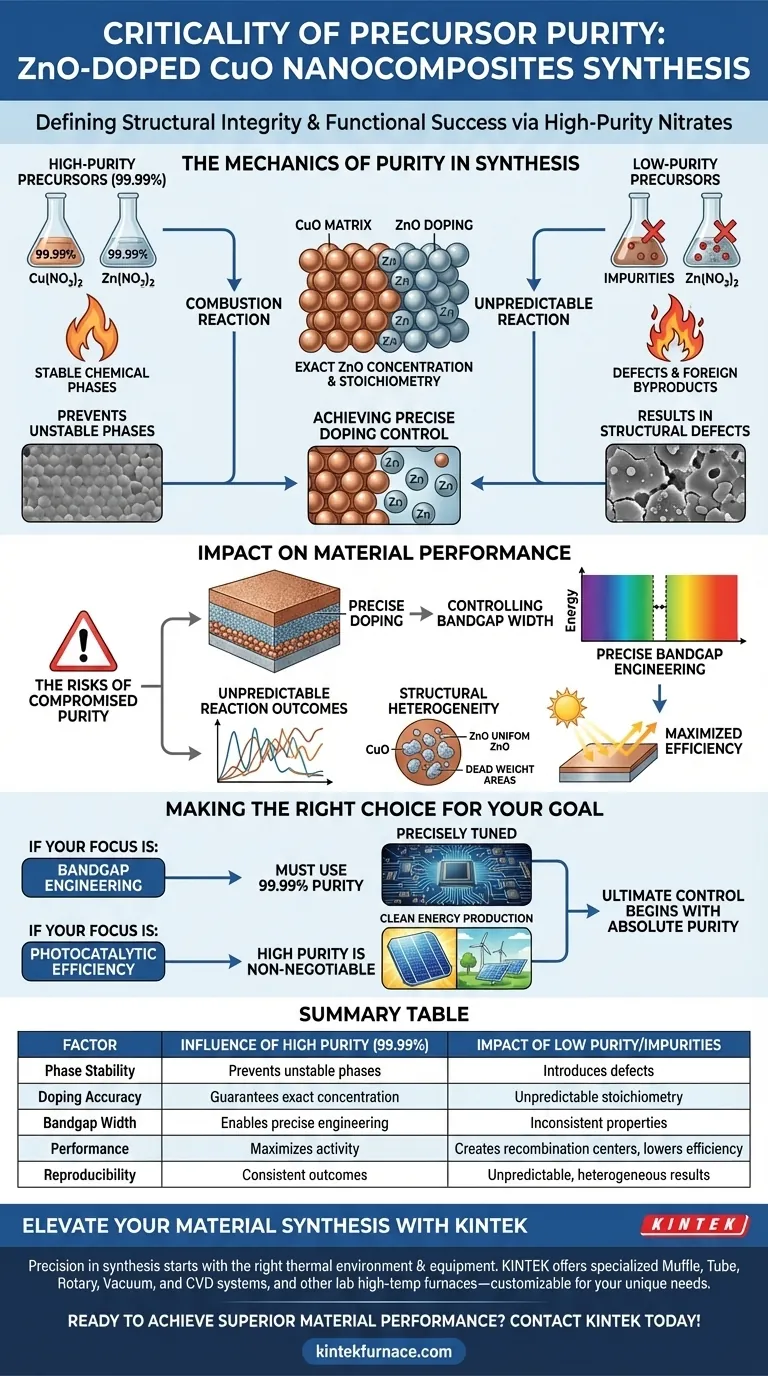
The purity of oxide precursors defines the structural integrity and functional success of ZnO-doped CuO nanocomposites. Specifically, utilizing high-purity nitrate precursors—typically at 99.99%—is required to prevent the introduction of impurities that disrupt the delicate combustion reaction. Without this rigorous standard, you cannot achieve the precise chemical stability necessary for effective doping.
High-purity inputs are the only way to avoid unstable chemical phases that degrade material performance. By strictly controlling precursor quality, you ensure precise ZnO doping concentrations, which directly dictate the bandgap width and the resulting photocatalytic efficiency of the nanocomposite.

The Mechanics of Purity in Synthesis
Preventing Unstable Chemical Phases
The synthesis of heterostructured nanocomposites is a sensitive chemical process. Using high-purity raw materials prevents the formation of unstable chemical phases during the combustion reaction.
If impurities are present, they can react unpredictably with the copper or zinc nitrates. This results in structural defects or foreign byproducts that compromise the stability of the final material.
Achieving Precise Doping Control
The core objective of this synthesis is to embed Zinc Oxide (ZnO) into a Copper Oxide (CuO) matrix.
High-purity precursors ensure that the ZnO doping concentration is exact. When you remove variable impurities from the equation, the ratio of reactants directly translates to the stoichiometry of the final product.
Impact on Material Performance
Controlling Bandgap Width
The physical properties of the nanocomposite are heavily dependent on how the ZnO interacts with the CuO.
The precision of the doping concentration directly influences the bandgap width of the material. Variations in purity lead to variations in doping, which causes inconsistent electronic properties.
Defining Photocatalytic Activity
For applications like environmental remediation or energy conversion, the material's ability to facilitate photoreactions is paramount.
Because purity dictates the bandgap, it subsequently controls the photocatalytic activity of the ZnO-doped CuO. A material synthesized with impure precursors will likely exhibit reduced efficiency in catalytic applications.
The Risks of Compromised Purity
Unpredictable Reaction Outcomes
When you utilize precursors below the 99.99% standard, you introduce variables that are difficult to account for.
The primary trade-off of lower purity is the loss of reproducibility. Impurities can alter the thermodynamics of the combustion reaction, leading to batch-to-batch inconsistencies that make scientific analysis impossible.
Structural Heterogeneity
Low-purity inputs often result in a heterogeneous material where the ZnO is not uniformly distributed within the CuO matrix.
This lack of uniformity creates areas of "dead weight" within the nanocomposite. These areas contribute to the mass of the material without contributing to its desired bandgap or photocatalytic properties.
Making the Right Choice for Your Goal
To ensure your ZnO-doped CuO nanocomposites perform as intended, select your materials based on the specific physical property you need to control.
- If your primary focus is Bandgap Engineering: You must use 99.99% purity precursors to guarantee the doping concentration matches your theoretical calculations.
- If your primary focus is Photocatalytic Efficiency: High purity is non-negotiable, as even minor impurities can create recombination centers that drastically lower activity.
Ultimate control over the final application begins with the absolute purity of the initial nitrates.
Summary Table:
| Factor | Influence of High Purity (99.99%) | Impact of Low Purity/Impurities |
|---|---|---|
| Phase Stability | Prevents unstable chemical phases | Introduces structural defects and foreign byproducts |
| Doping Accuracy | Guarantees exact ZnO concentration | Causes unpredictable stoichiometry and 'dead weight' |
| Bandgap Width | Enables precise bandgap engineering | Results in inconsistent electronic properties |
| Performance | Maximizes photocatalytic activity | Creates recombination centers that lower efficiency |
| Reproducibility | Consistent batch-to-batch outcomes | Leads to unpredictable and heterogeneous results |
Elevate Your Material Synthesis with KINTEK
Precision in your ZnO-doped CuO nanocomposites starts with the right thermal environment and high-quality equipment. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temp furnaces—all fully customizable to meet your unique synthesis needs. Whether you are focused on bandgap engineering or maximizing photocatalytic efficiency, our equipment ensures the stable, controlled conditions necessary for high-purity precursors to perform.
Ready to achieve superior material performance? Contact KINTEK today to discuss your custom furnace solution!
Visual Guide
References
- A. Naveen Kumar, Nithesh Naik. Solution combustion synthesis of ZnO doped CuO nanocomposite for photocatalytic and sensor applications. DOI: 10.1038/s41598-024-82764-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What role does physical homogenization via planetary ball milling play in LFP precursors? Maximize Your Battery Quality
- Why is a graphite furnace better than a flame in AAS? Unlock Trace-Level Detection for Your Lab
- What are the advantages of electric current-assisted TLP bonding? Maximize Efficiency for Inconel 718 Joining
- How does industrial-scale forging equipment influence the morphology of primary carbonitrides in H13 tool steel?
- What are the advantages of using a vacuum drying oven for ZIF67/MXene? Protect Your Composite Integrity
- Why is a high-precision blast drying oven used for Ni-Co/Ca catalyst preparation? Ensure Structural Integrity
- Why is precise temperature control necessary in a drying oven for MOF precursors? Ensure Nanopore Integrity
- Why is vacuum freeze-drying necessary for FeNC/MXene catalysts? Preserving 2D Architecture for Peak Performance



















