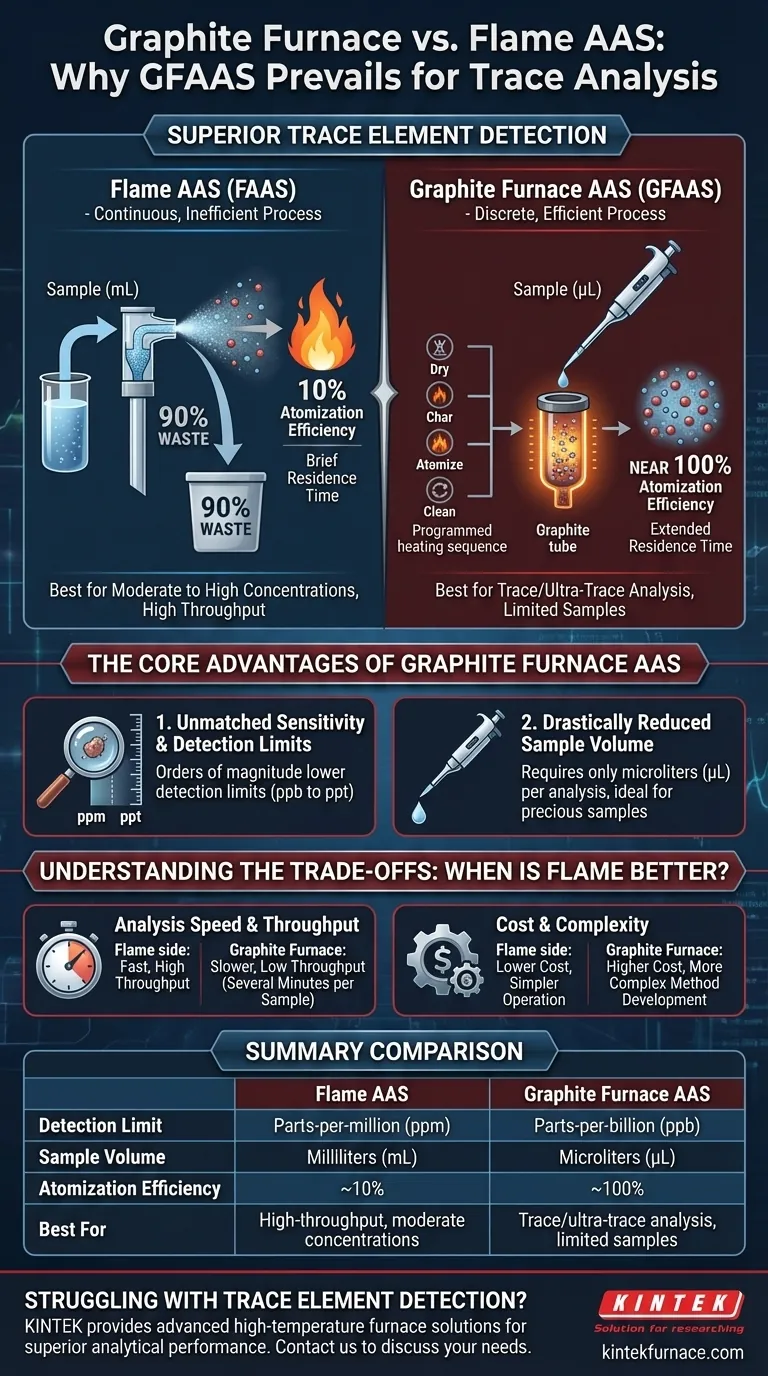
To put it simply, a graphite furnace is superior to a flame for atomic absorption spectroscopy (AAS) when your goal is to measure very low concentrations of an element. This superiority comes from two fundamental advantages: it atomizes nearly 100% of the injected sample and it confines the resulting cloud of atoms in the instrument's light path for several seconds, maximizing the signal.
While Flame AAS is a robust and fast technique for analyzing moderate to high concentrations, Graphite Furnace AAS (GFAAS) is the definitive choice for trace and ultra-trace analysis. Its superiority lies not in being universally better, but in its exceptional atomization efficiency and atomic containment, which translate directly into detection limits that are orders of magnitude lower.

The Core Difference: Atomization and Containment
To understand the performance gap, you must first understand how each technique turns a liquid sample into a measurable atomic gas. The efficiency of this process is the single most important factor.
Flame AAS: A Continuous, Inefficient Process
In Flame AAS (FAAS), the liquid sample is continuously aspirated into a spray chamber. Here, a nebulizer creates a fine aerosol.
Unfortunately, only about 10% of the original sample makes it to the flame as a usable aerosol. The other 90% condenses and goes to waste.
The atoms that are created in the flame then pass through the instrument's light path very quickly, giving the detector only a brief moment to perform its measurement.
Graphite Furnace AAS: A Discrete, Efficient Process
In Graphite Furnace AAS (GFAAS), a tiny, precise volume of the sample (typically in microliters) is injected directly into a graphite tube. This tube is then heated in a programmed sequence.
This process ensures that the entire sample is atomized, representing a near-perfect 100% efficiency. There is no waste.
The Power of Residence Time
The second key advantage is residence time. In GFAAS, the graphite tube temporarily traps the cloud of atomized sample.
Think of it like trying to count people. Flame AAS is like trying to count people as they flash past an open window. Graphite Furnace AAS is like having those same people stand still inside a room for several seconds while you count them.
This extended residence time allows the detector to measure the absorbance signal for a much longer period, dramatically improving the signal-to-noise ratio and overall measurement quality.
How This Translates to Performance
The radical differences in atomization efficiency and residence time have direct, practical consequences for your analytical results.
Unmatched Sensitivity and Detection Limits
Because it uses the entire sample and holds the atoms for a longer measurement, GFAAS produces a much stronger absorbance signal for the same concentration compared to FAAS.
This allows GFAAS to achieve detection limits that are 100 to 1,000 times lower than FAAS. While FAAS typically measures in the parts-per-million (ppm) range, GFAAS routinely measures in the parts-per-billion (ppb) range, and can even reach parts-per-trillion (ppt) for some elements.
Drastically Reduced Sample Volume
FAAS requires a continuous flow of sample to maintain a stable signal, often consuming several milliliters (mL) per element.
GFAAS is a discrete technique that requires only microliters (µL) of sample per analysis. This is a critical advantage when dealing with precious, biological, or limited-volume samples.
Understanding the Trade-offs: When is Flame Better?
A graphite furnace is not superior in every situation. Acknowledging its trade-offs is key to making an informed decision.
Analysis Speed and Throughput
A single GFAAS analysis takes several minutes due to the required heating program (drying, charring, atomizing, and cleaning). This results in low sample throughput.
FAAS provides a near-instantaneous and stable reading once the flame is running. It is the ideal choice for laboratories that need to process a high number of samples quickly.
Precision and Interferences
The high sensitivity and enclosed environment of the graphite furnace can make it more susceptible to background and chemical interferences, which requires more complex method development to overcome.
FAAS is generally considered a more rugged technique with better precision for higher concentration samples. It is less prone to certain types of interference.
Cost and Operational Complexity
GFAAS instruments are more expensive to purchase and operate. The graphite tubes are a consumable component with a limited lifetime of a few hundred firings, adding to the running costs.
Method development for GFAAS is also more complex and demands a higher level of operator skill compared to the relative simplicity of FAAS.
Making the Right Choice for Your Analysis
Your choice of technique should be driven entirely by your analytical goal.
- If your primary focus is trace element analysis or you have limited sample volume: GFAAS is the necessary and superior choice due to its unparalleled sensitivity.
- If your primary focus is routine analysis of major components or high sample throughput: FAAS is the more practical and cost-effective solution due to its speed and robustness.
- If you are analyzing elements at high concentrations (e.g., >10 ppm): FAAS is almost always the correct choice, as a GFAAS would be too sensitive and require massive dilutions that introduce error.
Choosing the right technique is not about which is universally "better," but which is precisely suited to your concentration range, sample matrix, and throughput needs.
Summary Table:
| Feature | Flame AAS | Graphite Furnace AAS |
|---|---|---|
| Detection Limit | Parts-per-million (ppm) | Parts-per-billion (ppb) |
| Sample Volume | Milliliters (mL) | Microliters (µL) |
| Atomization Efficiency | ~10% | ~100% |
| Best For | High-throughput, moderate concentrations | Trace/ultra-trace analysis, limited samples |
Struggling with trace element detection or limited sample volumes?
At KINTEK, we understand that precision in atomic spectroscopy is non-negotiable. Leveraging our exceptional R&D and in-house manufacturing, we provide advanced high-temperature furnace solutions, including specialized components for analytical instruments like AAS.
Our product line, featuring Muffle, Tube, and Vacuum Furnaces, is backed by strong deep customization capabilities. Whether you need robust furnace components or a tailored solution to meet unique experimental requirements, KINTEK is your partner in achieving superior analytical performance.
Elevate your lab's capabilities. Contact our experts today to discuss how we can support your precise analytical needs.
Visual Guide
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is a Quartz Tube Furnace and what is its primary function? Essential for Real-Time Material Observation
- What is the difference between an alumina tube furnace and a quartz tube furnace? Choose the Right Tube Furnace for Your Lab
- How should a quartz tube furnace be cleaned? Essential Steps for Safe, Contamination-Free Maintenance
- What is the necessity of using vacuum-sealed quartz tubes? Ensuring Integrity in Ti-Cu Alloy Heat Treatment
- What happens to convective and radiative heat transfer effects at high furnace gas temperatures? Radiation Dominates for Superior Heating



















