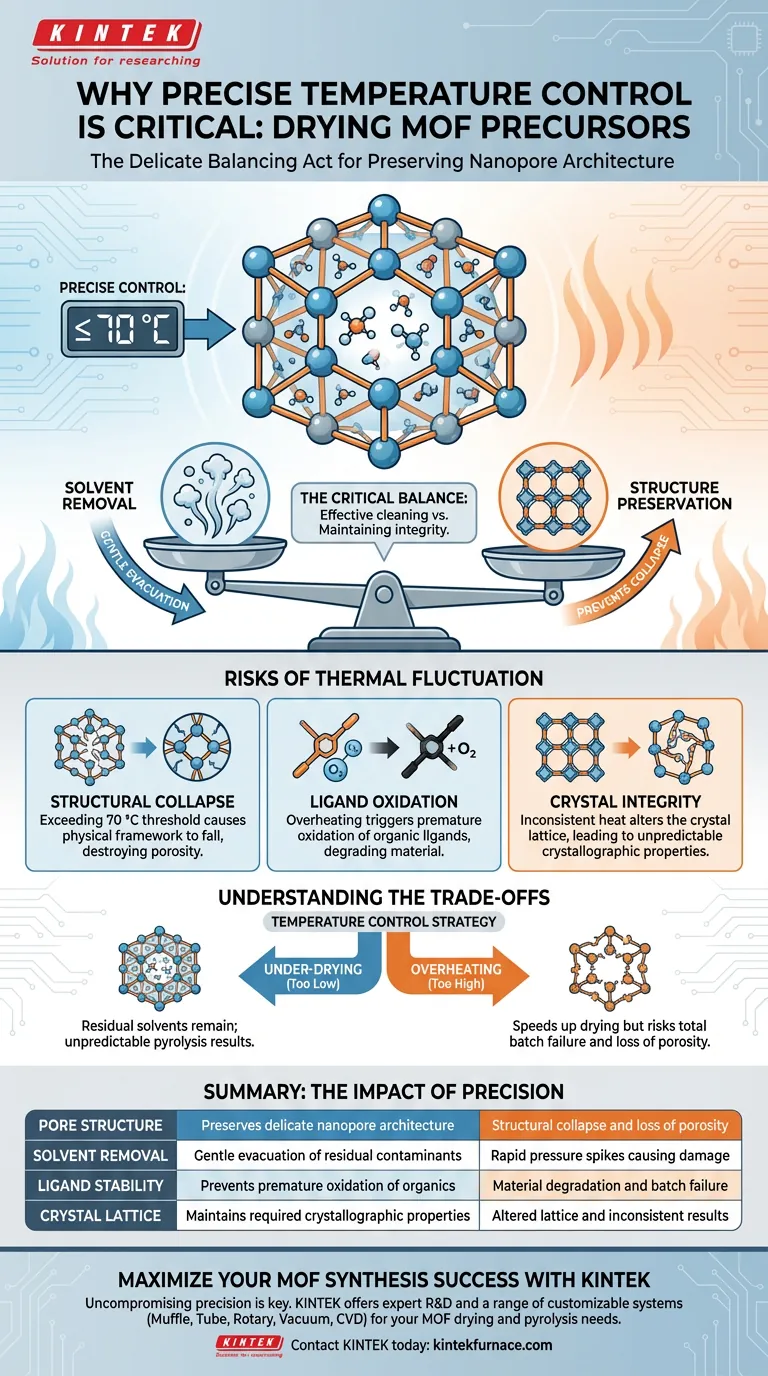
Precise temperature control is the single most critical factor in preserving the delicate architecture of Metal-Organic Framework (MOF) precursors during the drying phase. You must strictly maintain temperatures, typically at or below 70 °C, to effectively remove residual solvents and moisture from nanopores without triggering the thermal degradation of the material itself.
Core Takeaway The drying process is a balancing act between cleaning the pores and destroying the structure. Accurate thermal regulation ensures the MOF retains its specific crystal structure and intended porosity, preventing collapse or oxidation before it undergoes high-temperature pyrolysis.
The Critical Balance of Drying
Removing Contaminants
The primary function of the drying oven is the evacuation of residual solvents and moisture trapped within the MOF's nanopores.
Preserving Nanostructure
These solvents must be removed gently; rapid heating or temperature spikes can cause the internal pressure to rise too quickly, damaging the pore structure.
Setting the Stage for Pyrolysis
This drying phase is a preparatory step. By ensuring the precursor is contaminant-free and structurally sound now, you ensure the success of the subsequent high-temperature pyrolysis phase.
The Risks of Thermal Fluctuation
Preventing Structural Collapse
MOF precursors are thermally sensitive. If the temperature exceeds the specific threshold (often 70 °C), the physical framework holding the pores open can collapse.
Avoiding Ligand Oxidation
Precise control prevents overheating, which can lead to the oxidation of the organic ligands within the framework.
Maintaining Crystal Integrity
Fluctuations in temperature can alter the crystal lattice. Consistent heat ensures the material retains the exact crystallographic properties required for its end application.
Understanding the Trade-offs
The Risk of Under-drying
If the temperature is controlled too conservatively (too low), residual solvents remain. This can interfere with downstream processing, leading to unpredictable results during pyrolysis.
The Cost of Overheating
Conversely, pushing the temperature even slightly above the limit to speed up drying risks total batch failure. The trade-off for speed is often the destruction of the material's porosity—its most valuable attribute.
Making the Right Choice for Your Goal
To maximize the yield and quality of your MOF precursors, align your temperature strategy with your specific objectives:
- If your primary focus is Structural Integrity: Prioritize a lower, strictly regulated temperature ceiling (≤70 °C) to eliminate any risk of pore collapse or ligand damage.
- If your primary focus is Purity: Ensure the duration of the drying cycle is extended to compensate for the lower temperatures, guaranteeing complete solvent removal.
Success in MOF synthesis relies not on high heat, but on the precision of your control.
Summary Table:
| Factor | Impact of Precision | Risk of Poor Control |
|---|---|---|
| Pore Structure | Preserves delicate nanopore architecture | Structural collapse and loss of porosity |
| Solvent Removal | Gentle evacuation of residual contaminants | Rapid pressure spikes causing damage |
| Ligand Stability | Prevents premature oxidation of organics | Material degradation and batch failure |
| Crystal Lattice | Maintains required crystallographic properties | Altered lattice and inconsistent results |
Maximize Your MOF Synthesis Success with KINTEK
Preserving the delicate architecture of Metal-Organic Frameworks requires more than just heat—it requires uncompromising precision. At KINTEK, we understand that even a minor temperature fluctuation can compromise your research.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your specific MOF drying and pyrolysis needs.
Don't risk your batch integrity. Contact KINTEK today to find the precision heating solution tailored to your lab's unique requirements.
Visual Guide
References
- D. G. Muratov, А. В. Зорин. Metal-organic frameworks and composites on their basis: structure, synthesis methods, electrochemical properties and application prospects (a review). DOI: 10.3897/j.moem.10.2.126396
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the primary function of a high vacuum drying oven in B4C/Al powder pretreatment? Protect Purity & Prevent Pores
- Why is staged debinding necessary for perovskite ceramic green bodies? Prevent Cracking with Precision Control
- How do continuous furnaces differ from batch furnaces? Choose the Right Furnace for Your Production Needs
- What is the necessity of preheating reinforcement materials? Eliminate Defects in Aluminum Alloys
- How does precise heating rate control affect nitrogen-doped carbon synthesis? Master Thermal Ramp for Quality Materials
- How does the catalytic steam reforming system convert refinery waste gas into syngas for SOFC? Maximize Waste Energy
- What roles does a laboratory oven play in biochar production? Enhance Efficiency and Accuracy in Thermal Processing
- What role does a laboratory facility play in establishing the mass balance for a coke oven operation? Drive Efficiency.



















