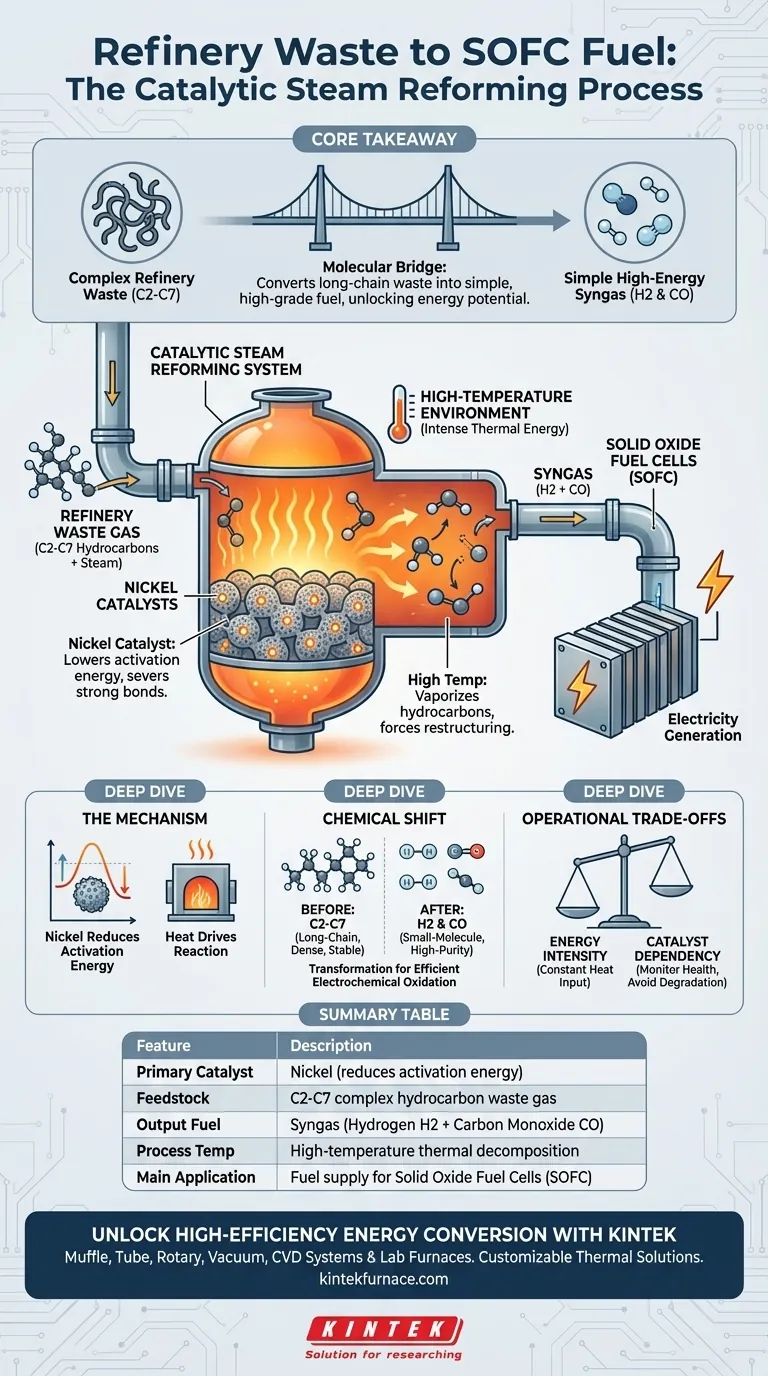
The catalytic steam reforming system functions by subjecting refinery waste gas to high temperatures in the presence of nickel catalysts. This intense thermal and chemical environment breaks down the complex hydrocarbon chains (C2-C7) found in the waste. The result is a recombined "syngas" mixture of hydrogen and carbon monoxide, transforming a waste byproduct into a high-grade fuel ready for direct use in Solid Oxide Fuel Cells (SOFC).
Core Takeaway: This system acts as a molecular bridge, converting difficult-to-use, long-chain refinery waste into simple, high-energy fuels. By stripping complex hydrocarbons down to their fundamental components (H2 and CO), it unlocks the full energy potential of waste gas for efficient electricity generation.

The Mechanism of Transformation
The Critical Role of Nickel Catalysts
The heart of this system relies on nickel catalysts to drive the chemical reaction. Without this catalyst, the chemical bonds holding the waste gas molecules together would remain stable.
The nickel lowers the activation energy required for the reaction. This allows the system to efficiently sever the strong bonds within the hydrocarbon chains.
Leveraging High-Temperature Environments
Chemical decomposition in this context is not a passive process; it requires a high-temperature environment. The system applies intense heat to the gas mixture to facilitate the reforming reaction.
This thermal energy ensures that the hydrocarbons are fully vaporized and reactive. It is the combination of heat and the nickel surface that forces the molecular restructuring.
From Waste to Fuel: The Chemical Shift
Breaking Down C2-C7 Hydrocarbons
Refinery waste gas typically consists of complex C2-C7 hydrocarbons. These are "long-chain" molecules that possess high calorific value but are chemically heavy.
These complex structures are often too dense or unstable for direct, efficient use in delicate power generation equipment. The reforming system specifically targets these chains for decomposition.
Creating High-Quality Syngas
The output of this process is syngas, a mixture primarily composed of hydrogen and carbon monoxide. Unlike the input gas, these are "small-molecule" fuels.
This transformation is fundamental for energy conversion. Small molecules like hydrogen and carbon monoxide are the preferred fuel sources for SOFCs, allowing for direct and highly efficient electrochemical oxidation.
Understanding the Operational Trade-offs
Energy Intensity
While effective, the requirement for high-temperature environments introduces an energy cost. Maintaining the thermal conditions necessary for reforming requires a constant input of heat.
Operators must balance the energy generated by the SOFC against the energy consumed to heat the reformer.
Catalyst Dependency
The system's reliance on nickel catalysts means performance is tied to catalyst health. If the catalyst degrades or becomes inactive, the conversion efficiency drops immediately.
This necessitates careful monitoring of the waste gas composition to ensure the catalyst remains effective over time.
Optimizing Waste-to-Energy Strategies
Implementing a catalytic steam reforming system is a strategic decision for refineries looking to valorize waste streams.
- If your primary focus is Waste Valorization: Prioritize this system to convert flared or vented C2-C7 waste gases into usable power generation assets.
- If your primary focus is SOFC Efficiency: Use this reforming step to ensure your fuel cells receive the high-purity, small-molecule feedstock (H2 and CO) they require for maximum output.
By effectively reducing molecular complexity, you turn an environmental liability into a valuable energy resource.
Summary Table:
| Feature | Description |
|---|---|
| Primary Catalyst | Nickel (reduces activation energy) |
| Feedstock | C2-C7 complex hydrocarbon refinery waste gas |
| Output Fuel | Syngas (Hydrogen H2 + Carbon Monoxide CO) |
| Process Temp | High-temperature thermal decomposition |
| Main Application | Fuel supply for Solid Oxide Fuel Cells (SOFC) |
Unlock High-Efficiency Energy Conversion with KINTEK
Transform your refinery by-products into high-value energy resources. Backed by expert R&D and precision manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your specific catalytic reforming and SOFC research needs.
Our advanced thermal solutions ensure the precise temperature control and reliability required to break down complex hydrocarbons and optimize syngas production. Contact us today to discover how our high-temperature technology can power your next breakthrough in waste-to-energy valorization.
Visual Guide
References
- Ivan Beloev, Iliya Iliev. Utilization of Hydrogen-Containing Gas Waste from Deep Oil Refining at a Hybrid Power Plant with a Solid Oxide Fuel Cell. DOI: 10.3390/engproc2024060005
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How does the design of specialized industrial furnaces for hydrogen production contribute to extension of lifespan?
- Why is the precision of an automatic temperature-controlled furnace critical in glass synthesis? Achieve 1350°C Accuracy
- What is the primary purpose of using nano-magnesium oxide as a template? Optimize Sulfur-Doped Porous Carbon Synthesis
- What are the three steps parts go through in a conveyor furnace? Master Sintering for Stronger Parts
- Why is the selection of electrode materials critical for the Plasma Flash Sintering (PFS) of titanium dioxide samples?
- What is the function of an industrial electric furnace in Al-Cu 224 alloy preparation? Optimize Your Metal Production
- Importance of NaH2PO2 Layout in V-Ni3S2/NF Phosphorization: Ensuring Uniform 3D Doping
- How does a programmable high-temperature furnace improve the control of cooling rates? Enhance Ceramic Part Integrity



















