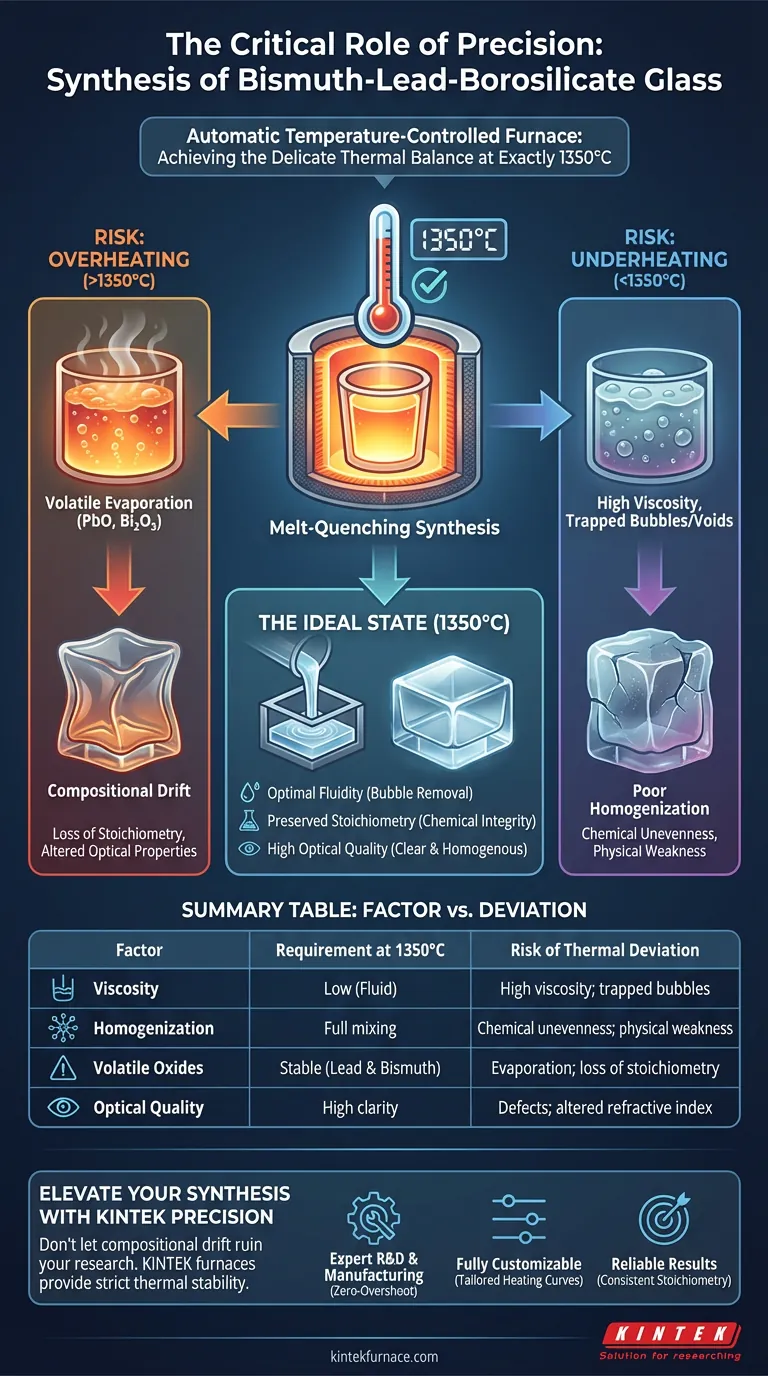
Precision in thermal regulation is the defining factor in the successful synthesis of bismuth-lead-borosilicate glass. An automatic temperature-controlled furnace is critical because it maintains the melt exactly at 1350°C, ensuring the material becomes fluid enough to release trapped gas bubbles without overheating to the point where volatile components evaporate.
The synthesis of this glass requires a delicate thermal balance: the temperature must be high enough to lower viscosity for homogenization and bubble removal, yet strictly controlled to prevent the loss of volatile lead and bismuth oxides, thereby preserving the material's intended chemical composition.

Achieving the Delicate Thermal Balance
The Necessity of Optimal Fluidity
To create high-quality glass, the raw materials must reach a state of optimal fluidity.
In the bismuth-lead-borosilicate system, this occurs specifically at 1350°C. At this temperature, the viscosity of the melt drops sufficiently to allow trapped air bubbles to rise to the surface and escape.
Without reaching this precise threshold, the final glass would likely contain defects and voids, compromising its structural and optical quality.
Controlling Volatile Components
While high heat is necessary for fluidity, it presents a significant risk to the chemical makeup of the glass.
This specific glass system contains lead oxide and bismuth oxide, both of which are highly volatile components. If the temperature exceeds the required parameters, these oxides will begin to evaporate from the melt.
An automatic furnace prevents temperature spikes, ensuring the heat remains constant rather than fluctuating into dangerous ranges where evaporation occurs.
Preserving Chemical Integrity
Maintaining Stoichiometry
The ultimate goal of the melt-quenching technique is to produce a glass that matches a specific chemical formula.
The stoichiometric ratio—the precise proportion of elements in the final product—depends entirely on preventing the loss of raw materials during heating.
By strictly regulating the heating curves, the furnace ensures that the amount of lead and bismuth put into the mix remains in the final glass, rather than being lost to the atmosphere.
Understanding the Risks of Thermal Deviation
The Consequence of Overheating
If the furnace control fails and the temperature rises too high, you face immediate compositional drift.
The evaporation of volatile oxides alters the glass's refractive index and density. Once these components vaporize, the final product will no longer meet the design specifications.
The Consequence of Underheating
Conversely, failing to maintain the target temperature results in poor homogenization.
If the melt is too cool, it remains too viscous. This prevents bubbles from escaping (fining) and stops the various components from mixing thoroughly, leading to a chemically uneven and physically weak material.
Making the Right Choice for Your Synthesis
To ensure the reproducibility of your bismuth-lead-borosilicate glass, consider these operational priorities:
- If your primary focus is Optical Clarity: Ensure the furnace can hold 1350°C consistently to minimize viscosity and maximize bubble removal.
- If your primary focus is Chemical Accuracy: Verify that the furnace has strict overshoot protection to prevent the vaporization of lead and bismuth oxides.
True precision in synthesis is not just about reaching a temperature; it is about maintaining the specific environment where chemistry and physics align perfectly.
Summary Table:
| Factor | Requirement at 1350°C | Risk of Thermal Deviation |
|---|---|---|
| Viscosity | Low (Fluid) | High viscosity; trapped bubbles/voids |
| Homogenization | Full mixing | Chemical unevenness; physical weakness |
| Volatile Oxides | Stable (Lead & Bismuth) | Evaporation; loss of stoichiometry |
| Optical Quality | High clarity | Defects; altered refractive index |
Elevate Your Glass Synthesis with KINTEK Precision
Don't let compositional drift or poor homogenization ruin your specialized glass research. KINTEK provides high-performance furnace solutions—including Muffle, Tube, and Vacuum systems—specifically designed to maintain the strict thermal stability required for volatile materials like bismuth-lead-borosilicate.
Our Value to You:
- Expert R&D & Manufacturing: Systems engineered for zero-overshoot protection.
- Fully Customizable: Tailored heating curves and atmosphere control for your unique synthesis needs.
- Reliable Results: Ensure the stoichiometry and optical clarity of every batch.
Ready to achieve perfect thermal regulation? Contact KINTEK today for a customized quote!
Visual Guide
References
- M. Gopi Krishna, N V Prasad. Characterization of a Novel System of Bismuth Lead Borosilicate Glass Containing Copper. DOI: 10.17485/ijst/v17i9.81
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is precise heating rate control necessary during the pyrolysis of bamboo? Optimize Au-NPs/BC Nanocomposite Quality
- What are the methods of heat transfer in furnaces? Master Heat Control for Better Results
- What is the purpose of the constant-temperature circulation phase? Ensure Moso Bamboo Integrity with KINTEK Solutions
- What are the complexities and maintenance requirements of continuous furnaces? Optimize High-Volume Production with Expert Insights
- Why is a slow heating rate utilized for rice husk biochar? Optimize Pore Structure and Adsorption Performance
- Why is precise control of heating and cooling rates necessary for iron-doped ceria? Optimize Your Catalyst Performance
- How does the carbon reductant ratio influence the selective reduction of ferronickel? Mastering Alloy Purity
- How do digital technical summaries assist the scientific community? Unlocking Lab High-Temp Furnace Insights



















