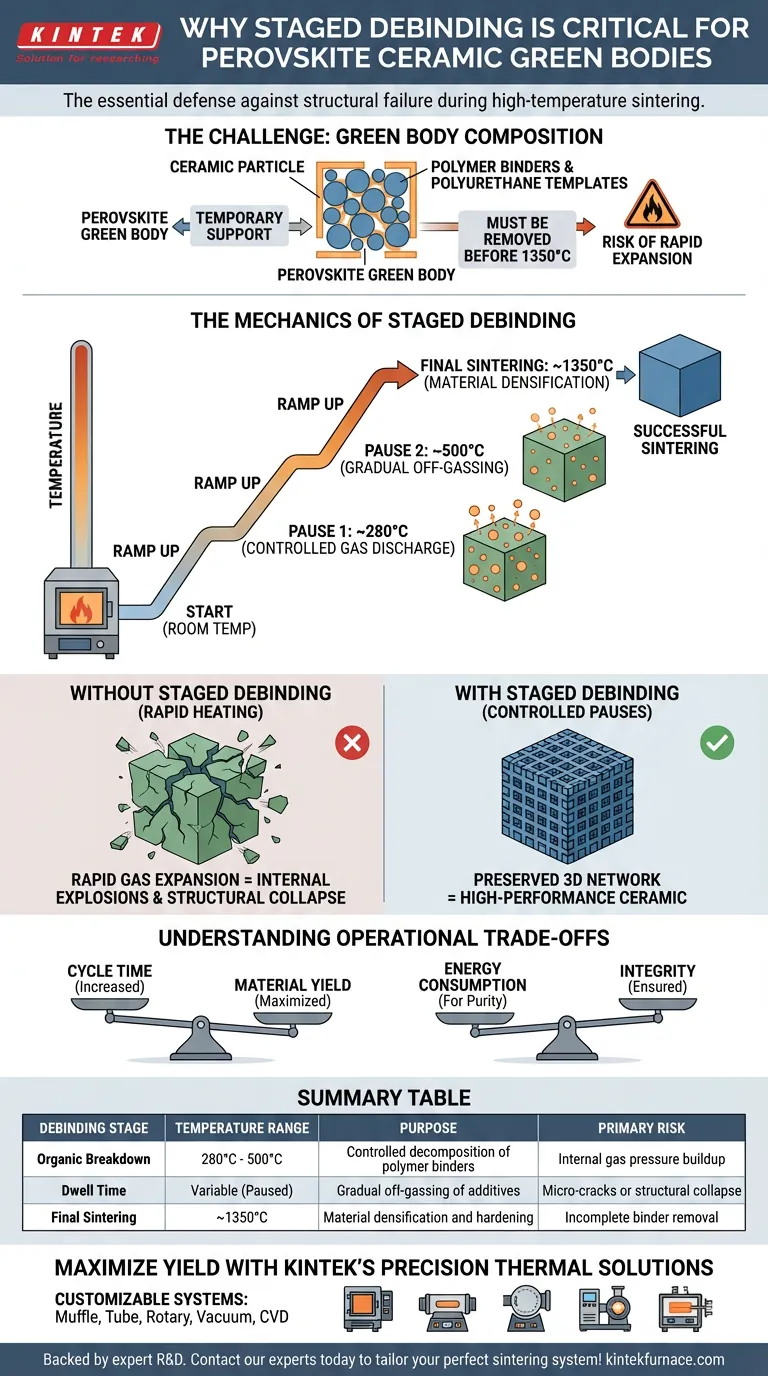
Staged debinding is the critical defense mechanism against structural failure during the processing of perovskite ceramic green bodies. Because these materials contain significant amounts of polymer binders and polyurethane templates, heating them directly to high sintering temperatures without pauses would cause rapid gas expansion, leading to the collapse or cracking of the material's internal structure.
By implementing a staged heating profile, you allow organic additives to decompose and exit the material gradually. This controlled release prevents internal pressure buildup, preserving the delicate three-dimensional network of the ceramic before it reaches final densification.
The Challenge of Green Body Composition
The Role of Organic Additives
Perovskite green bodies are not composed solely of ceramic material prior to firing. They rely on a structural matrix consisting of polymer binders and polyurethane templates.
Temporary Support, Permanent Risk
These organic components are essential for shaping the green body, but they become liabilities at high temperatures. They must be completely removed before the material reaches its final sintering temperature of 1350 °C.
The Mechanics of Staged Debinding
Targeting Specific Decomposition Points
The debinding process is not a linear ramp; it is a series of calculated pauses. The furnace is programmed to hold at specific intervals, typically around 280 °C and 500 °C.
Controlled Gas Discharge
These specific temperature plateaus match the decomposition characteristics of the binders. By holding at these temperatures, the organic matter breaks down slowly rather than flashing into gas instantly.
Preventing Structural Failure
The Danger of Rapid Expansion
If the temperature ramps up too quickly, the solid organic material converts to gas at an uncontrollable rate. This creates massive internal pressure within the ceramic body.
Preserving the 3D Network
This rapid gas release acts like a series of internal explosions. Without staged debinding, this pressure causes the three-dimensional network structure to crack or collapse entirely.
Understanding the Operational Trade-offs
Cycle Time vs. Material Yield
Implementing staged debinding significantly increases the total time required for a sintering cycle. You are trading process speed for material survival; skipping these stages to save time almost invariably leads to a wasted batch.
Energy Consumption vs. Integrity
Holding the furnace at 280 °C and 500 °C requires energy expenditure without densifying the ceramic. However, this "wasted" energy is the necessary cost of ensuring the green body is pure and stable enough to withstand the final ramp to 1350 °C.
Making the Right Choice for Your Goal
To ensure the successful production of perovskite ceramics, you must tailor your thermal profile to the chemistry of your binders.
- If your primary focus is maximizing yield: Extend the dwell times at 280 °C and 500 °C to guarantee that even thick sections of the green body have fully off-gassed.
- If your primary focus is cycle optimization: Experiment to find the minimum dwell time required at these stages, but never eliminate the pauses entirely.
A patient, precision-controlled preheating phase is the difference between a high-performance ceramic and a pile of broken fragments.
Summary Table:
| Debinding Stage | Temperature Range | Purpose | Primary Risk |
|---|---|---|---|
| Organic Breakdown | 280°C - 500°C | Controlled decomposition of polymer binders | Internal gas pressure buildup |
| Dwell Time | Variable (Paused) | Gradual off-gassing of additives | Micro-cracks or structural collapse |
| Final Sintering | ~1350°C | Material densification and hardening | Incomplete binder removal |
Maximize your material yield and structural integrity with KINTEK’s precision thermal solutions. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to handle the delicate staged heating profiles required for perovskite ceramics. Whether you need precise atmospheric control or specialized high-temp furnace configurations, our team ensures your lab achieves superior results. Contact our experts today to tailor the perfect sintering system for your unique research needs!
Visual Guide
References
- Mathias Pein, Christian Sattler. Thermochemical Oxygen Pumping with Perovskite Reticulated Porous Ceramics for Enhanced Reduction of Ceria in Thermochemical Fuel Production. DOI: 10.1002/aenm.202304454
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- Why is MFI-type zeolite (S-1) selected for H-TiO2 synthesis? Master High-Efficiency Nanoparticle Templating
- Why is it necessary to connect a pyrolyser online with a GC-MS? Achieve High-Fidelity RDF Analysis
- What is the necessity of baking electrode sheets in a vacuum oven? Ensure Battery Stability and Peak Performance
- Why is the calcination step essential for Copper Ferrite? Unlock High Purity & Superior Crystallinity
- What is the role of temperature control in MCM-41 synthesis? Master Precision Pore Engineering
- How does an electric furnace ensure accurate gasification? Master Isothermal and Dynamic Thermal Control
- Why is high-temperature stability important for Cu2O substrates? Ensure Long-Term Electrocatalytic Efficiency
- What are the core technical advantages of using SPS for Titanium Diboride ceramics? Achieve High Density & Fine Grains



















