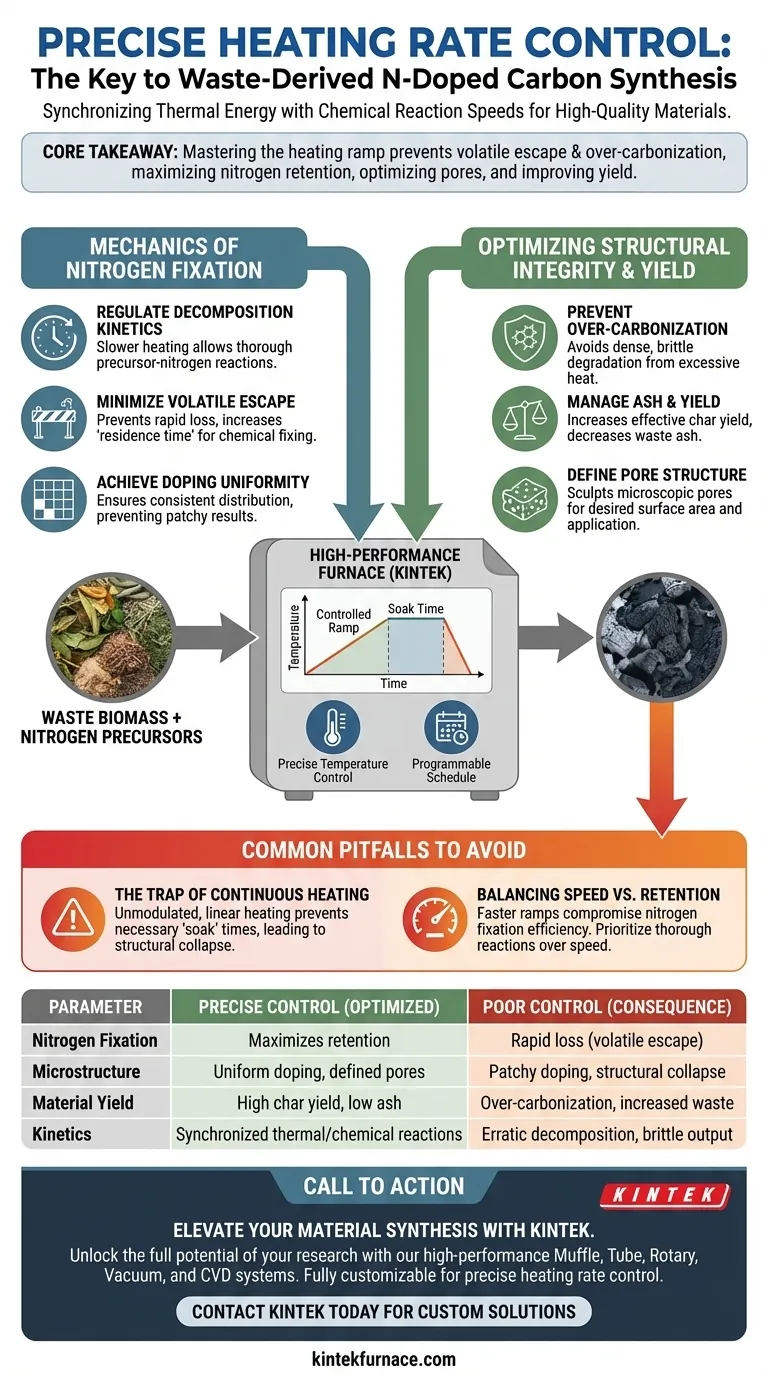
Precise heating rate control dictates the fundamental chemistry of carbonization. In the synthesis of waste-derived nitrogen-doped carbon, this control regulates the decomposition kinetics of biomass, ensuring that nitrogen atoms are effectively fixed into the carbon lattice rather than being lost during rapid volatilization. By managing the thermal ramp, you directly optimize the material's microstructure and ensure uniform nitrogen distribution.
Core Takeaway Mastering the heating ramp is not just about reaching a target temperature; it is about synchronizing thermal energy with chemical reaction speeds. Precise control prevents the rapid escape of volatiles and over-carbonization, thereby maximizing nitrogen retention, optimizing pore structure, and improving the overall yield of high-quality activated carbon.

The Mechanics of Nitrogen Fixation
Regulating Decomposition Kinetics
The primary benefit of a high-performance furnace is the ability to dictate exactly how fast the temperature rises.
Decomposition kinetics—the speed at which chemical bonds break—are highly sensitive to this rate.
By utilizing a slower, controlled heating rate, you allow for more thorough and complete reactions between the carbon precursors and the nitrogen sources.
Minimizing Volatile Escape
When biomass is heated too quickly, volatile components often flash into gas and escape the material immediately.
This rapid exit is detrimental to doping because it carries potential nitrogen atoms away before they can bond with the carbon.
Precise control slows this process, reducing the rapid escape of volatile matter and increasing the "residence time" for nitrogen to be chemically fixed into the structure.
Achieving Doping Uniformity
The ultimate goal of nitrogen doping is to alter the electronic properties of the carbon.
If the heating is erratic or too fast, the doping becomes patchy.
Controlled thermal processing ensures the overall uniformity of the nitrogen doping, creating a consistent material that performs reliably in catalytic or storage applications.
Optimizing Structural Integrity and Yield
Preventing Over-Carbonization
Beyond the chemical composition, the physical structure of the carbon is at risk during synthesis.
Without precise control, or when using simple continuous heating, the biomass can suffer from over-carbonization.
This state represents a degradation of the material quality, where the carbon structure becomes too dense or brittle, losing its functional value.
Managing Ash and Yield
Efficiency is a critical metric in waste-derived synthesis.
Lack of control correlates directly with increased ash production, which is effectively waste product within your waste-derived material.
Furthermore, uncontrolled heating reduces the effective char yield, meaning you produce less usable activated carbon from your initial raw material.
Defining Pore Structure
The utility of activated carbon often lies in its surface area and porosity.
The microscopic pore structure is not accidental; it is sculpted by the heating ramp.
Using an experimental furnace with programmable temperature control is decisive for optimizing these pores, ensuring the material has the necessary surface area for its intended application.
Common Pitfalls to Avoid
The Trap of Continuous Heating
A common mistake is assuming that "heating up" is a linear, passive process.
Continuous, unmodulated heating prevents the necessary "soak" times or slow ramps required for complex organic reactions.
This approach frequently leads to structural collapse and low-quality output.
Balancing Speed vs. Retention
There is a trade-off between processing speed and material quality.
While a faster ramp is desirable for production throughput, it almost invariably compromises nitrogen fixation efficiency.
The objective is to find the maximum rate that still permits thorough precursor reactions, rather than simply heating as fast as the furnace allows.
Making the Right Choice for Your Goal
To maximize the quality of your waste-derived carbon, you must tailor your furnace programming to your specific objectives.
- If your primary focus is Nitrogen Content: Prioritize a slower heating rate to maximize precursor interaction and minimize the loss of volatile nitrogen species.
- If your primary focus is Material Yield: Use programmable control to strictly limit the upper temperature and ramp speed to prevent over-carbonization and excess ash formation.
- If your primary focus is Pore Architecture: Utilize complex ramp schedules that prevent rapid volatilization, allowing pores to develop without collapsing the carbon skeleton.
Precise thermal regulation transforms biological waste into sophisticated functional materials by aligning the heating process with the material's chemical needs.
Summary Table:
| Parameter Optimized | Impact of Precise Control | Consequence of Poor Control |
|---|---|---|
| Nitrogen Fixation | Maximizes retention in the carbon lattice | Rapid loss through volatile escape |
| Microstructure | Uniform doping and defined pore architecture | Patchy doping and structural collapse |
| Material Yield | High effective char yield; low ash | Over-carbonization and increased waste |
| Kinetics | Synchronized thermal/chemical reactions | Erratic decomposition and brittle output |
Elevate Your Material Synthesis with KINTEK
Unlock the full potential of your carbon research with high-performance thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all precision-engineered to provide the exact heating rate control required for nitrogen doping and biomass conversion.
Whether you need to optimize pore architecture or maximize chemical yield, our lab high-temp furnaces are fully customizable to meet your unique experimental needs.
Ready to achieve superior nitrogen retention and material uniformity?
Contact KINTEK today to find your custom furnace solution!
Visual Guide
References
- Xing Huang, Dessie Ashagrie Tafere. Waste-derived green N-doped materials: mechanistic insights, synthesis, and comprehensive evaluation. DOI: 10.1039/d5su00555h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is the function of a stainless steel high-pressure reactor in HTC? Optimize Ion-Exchange Resin Conversion
- What is the purpose of using an industrial-grade drying oven to heat wood samples to 103 °C? Enhance Resin Impregnation
- What is the specific function of laboratory electric heating devices in solid-state hydrogen storage? Optimize Thermal Management
- Why is titanium used as a gettering agent in TiCo1-xCrxSb preparation? Achieve Purity in Your Alloy Synthesis
- How does a high-precision temperature control system influence the nanoparticle size? Master Catalyst Activation
- What are the advantages of PVD? Achieve High-Performance, Durable Coatings
- What is the significance of calculating AC impedance in the power control of indirect heating resistance furnaces?
- Why is it necessary for sintering equipment to have a high-cooling-rate control for 17-4 PH? Master Your Metallurgy



















