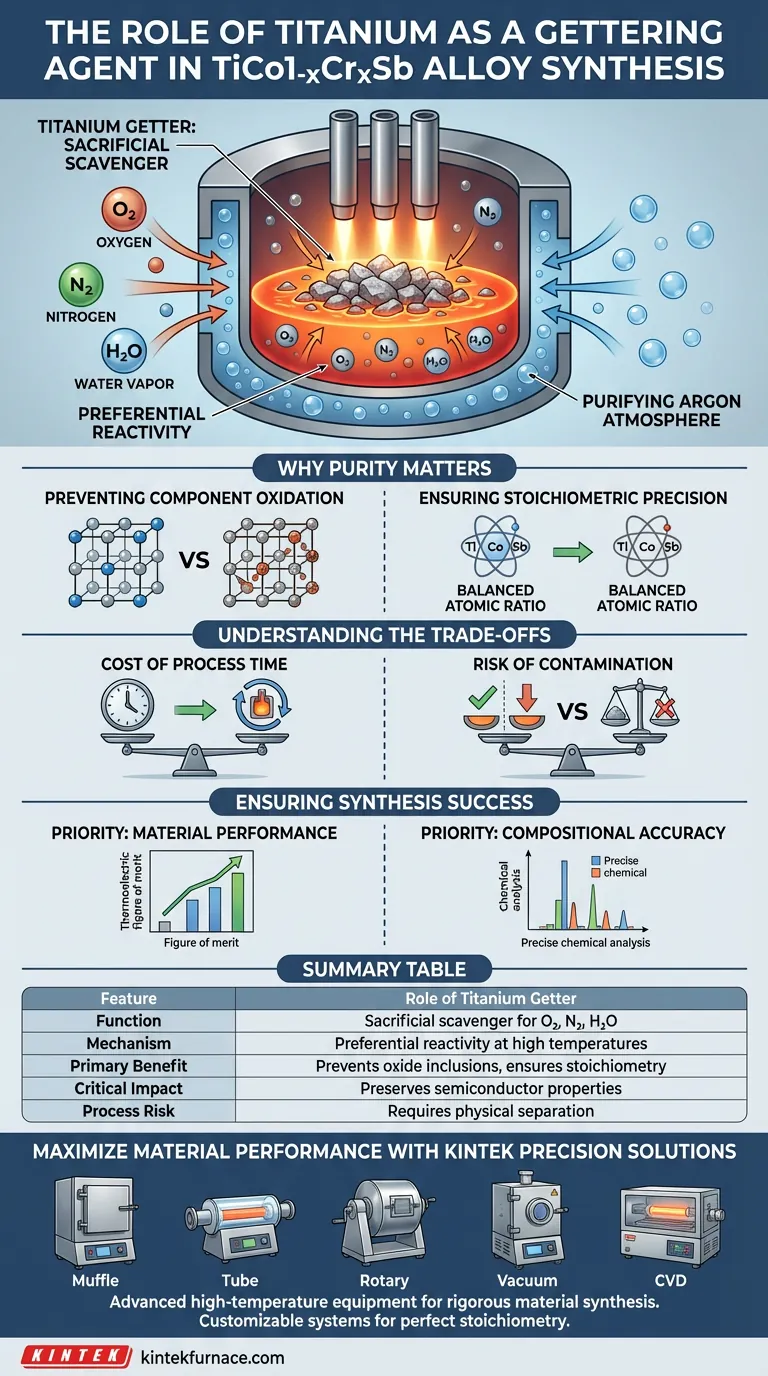
Titanium serves as a sacrificial scavenger designed to purify the melting environment before the actual alloy synthesis begins. During the preparation of TiCo1-xCrxSb alloys in an arc furnace, titanium sponge or chips are melted to react preferentially with residual oxygen, nitrogen, and water vapor. This process effectively strips these impurities from the argon atmosphere, preventing them from contaminating the sensitive main alloy.
By eliminating trace atmospheric gases, titanium gettering ensures the precise stoichiometric ratio required for high-performance thermoelectric materials. Without this step, oxidation would alter the alloy's composition and degrade its physical properties.

The Mechanics of Gettering
Preferential Reactivity
Titanium possesses an extremely high chemical affinity for oxygen and nitrogen at elevated temperatures. When melted, it acts as a "trap," reacting with these gases much faster than the other components in the furnace can.
Purifying the Argon Atmosphere
Even high-purity argon gas sources can contain trace amounts of contaminants that are detrimental to sensitive alloys. The molten titanium acts as a final filtration step within the chamber. It essentially "scrubs" the gas environment, ensuring that the argon surrounding your sample is truly inert.
Why Purity Matters for TiCo1-xCrxSb
Preventing Component Oxidation
The elements within the TiCo1-xCrxSb matrix are susceptible to oxidation at the high temperatures required for arc melting. If oxygen is present, it will react with the alloy components to form unwanted oxides. This results in inclusions that act as defects, scattering electrons and phonons in unpredictable ways.
Ensuring Stoichiometric Precision
Thermoelectric performance relies heavily on maintaining a specific atomic ratio (stoichiometry). If a portion of your titanium or cobalt is consumed by oxidation, the actual composition of the alloy shifts away from the target formula. This shift can destroy the semiconducting properties that make the material useful.
Understanding the Trade-offs
The Cost of Process Time
Using a titanium getter adds a distinct step to the manufacturing process. You must melt the getter material first and allow it to scavenge the atmosphere before introducing heat to your main sample. This requires patience and precise control of the arc manipulator to avoid rushing the purification phase.
Risk of Contamination
While the goal is purity, the getter itself can become a source of contamination if mishandled. If the arc melts the getter and then immediately touches the main alloy charge without cleaning the electrode or moving the hearth correctly, you risk introducing excess titanium into your formula. This would unintentionally alter the stoichiometry you are trying to protect.
Ensuring Synthesis Success
The use of titanium gettering is not merely a precautionary step; it is a fundamental requirement for producing semiconductor-grade half-Heusler alloys.
- If your primary focus is material performance: Prioritize a thorough gettering melt cycle to minimize oxide inclusions and maximize the thermoelectric figure of merit.
- If your primary focus is compositional accuracy: Ensure the getter is physically separated from the main charge in the hearth to prevent cross-contamination during the melting process.
Mastering the gettering step is the difference between creating a high-efficiency electronic material and a defective metallic slug.
Summary Table:
| Feature | Role of Titanium Getter in Alloy Synthesis |
|---|---|
| Function | Sacrificial scavenger for O₂, N₂, and H₂O |
| Mechanism | Preferential reactivity at high temperatures to 'scrub' atmosphere |
| Primary Benefit | Prevents oxide inclusions and ensures stoichiometric precision |
| Critical Impact | Preserves semiconductor properties and thermoelectric efficiency |
| Process Risk | Requires physical separation to avoid cross-contamination |
Maximize Material Performance with KINTEK Precision Solutions
Don't let trace impurities compromise your research. KINTEK provides the advanced high-temperature equipment needed for rigorous material synthesis. Backed by expert R&D and manufacturing, we offer a wide range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the exacting demands of thermoelectric alloy preparation.
Whether you need precise atmospheric control or robust vacuum capabilities, our lab furnaces are designed to help you achieve perfect stoichiometry every time. Contact us today to find the perfect furnace for your lab!
Visual Guide
References
- Volodymyr Krayovskyy, А. Horyn. SIMULATION OF CHARACTERISTICS OF SENSITIVE ELEMENTS OF TEMPERATURE CONVERTERS BASED ON TiCo1-xCrxSb. DOI: 10.23939/istcmtm2024.04.030
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
People Also Ask
- How does plant metal-ion absorption influence pyrolysis? Enhance Material Synthesis with Biological Pretreatment
- Why is the initial concentration of siloxane systems performed in a vacuum oven? Achieve Defect-Free Material Curing
- Why must temperature loss be monitored during the aluminum alloy refining cycle? Essential Tips for Casting Success
- What are the main types of laboratory furnaces based on size? Find the Perfect Fit for Your Lab's Scale
- What are the advantages of HTL reactors for algae? Optimize Biomass Conversion Without Pre-Drying
- What is Skin Depth and how does it affect induction heating? Master Frequency Control for Precise Heat
- What is the primary purpose of 24-hour wet milling for SSBSN ceramics? Achieve Atomic-Scale Homogeneity
- How does a precision temperature-controlled furnace facilitate the long-term aging treatment of Invar 36?
