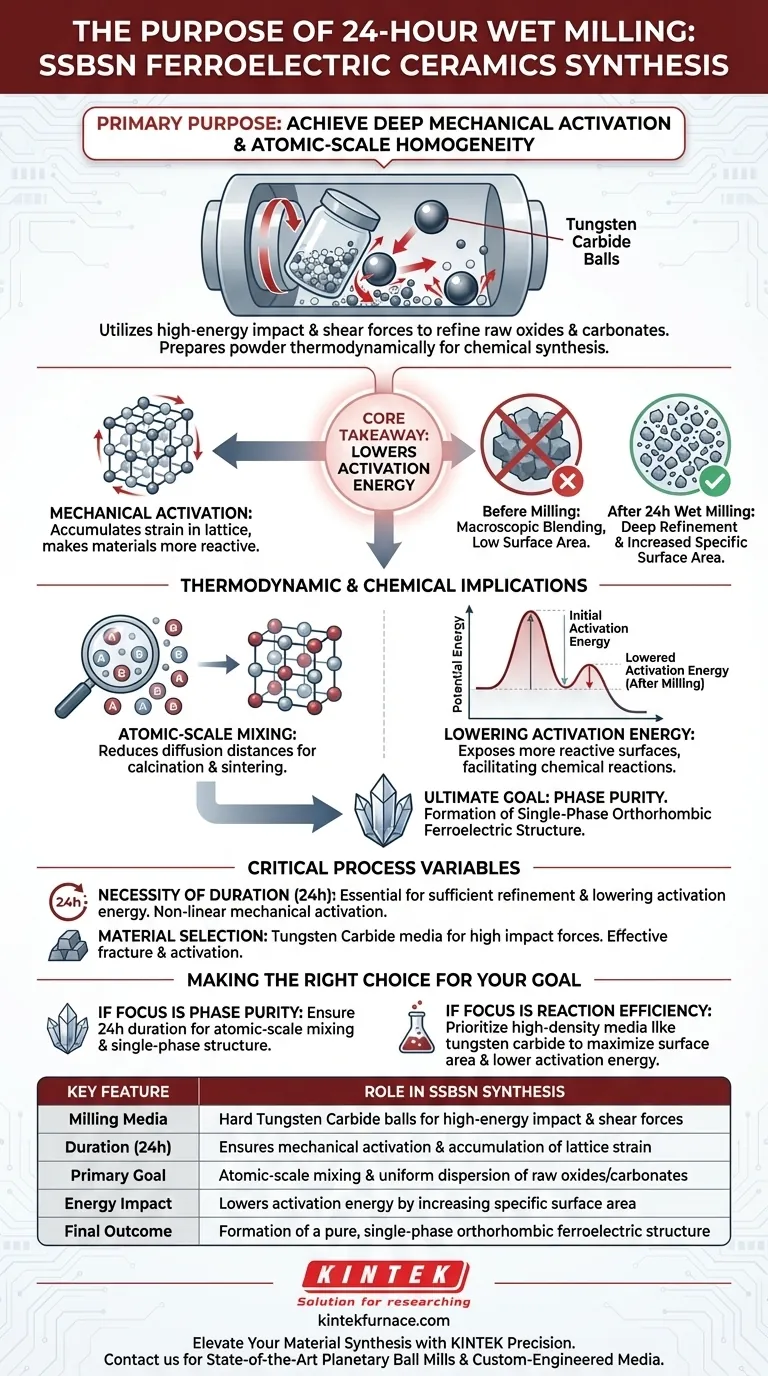
The primary purpose of conducting 24-hour wet milling is to achieve deep mechanical activation and atomic-scale homogeneity of the raw materials.
This process utilizes high-energy impact and shear forces to refine raw oxides and carbonates far beyond simple particle size reduction. By subjecting the mixture to prolonged grinding with hard tungsten carbide alloy balls, the process prepares the powder thermodynamically for successful chemical synthesis.
Core Takeaway While particle reduction is a visible result, the critical function of 24-hour wet milling is lowering the activation energy of the system. This step increases specific surface area and ensures uniform mixing, which is the absolute prerequisite for forming a pure, single-phase orthorhombic structure in the final ceramic.
The Mechanics of Structural Refinement
Utilization of High-Energy Forces
The planetary ball mill operates by generating significant kinetic energy. It relies on the collision of hard tungsten carbide alloy balls to deliver intense impact and shear forces to the raw powder.
Mechanical Activation
This 24-hour duration is not arbitrary; it is necessary to induce mechanical activation. This involves accumulating strain in the lattice of the raw materials, making them more reactive and ready for chemical transformation.
Deep Refinement
The process targets the raw carbonate and oxide materials. It breaks down agglomerates and fractures particles to achieve a level of refinement that standard mixing cannot replicate.
Thermodynamic and Chemical Implications
Atomic-Scale Mixing
For complex ceramics like SSBSN, macroscopic blending is insufficient. Wet milling ensures that the chemical components are mixed uniformly at the atomic scale, reducing the diffusion distances required during calcination and sintering.
Lowering Activation Energy
By drastically increasing the specific surface area of the powder, the milling process exposes more reactive surfaces. This directly lowers the activation energy required for the subsequent chemical reactions to occur.
Promoting Phase Purity
The ultimate goal of these thermodynamic adjustments is to facilitate a specific crystal structure. The prepared powder is optimized to form a single-phase orthorhombic structure, which is essential for the material's ferroelectric properties.
Critical Process Variables
The Necessity of Duration
The specific requirement of a 24-hour cycle highlights the non-linear nature of mechanical activation. Shortening this timeframe risks insufficient refinement, leaving the activation energy too high to achieve phase purity during later heating stages.
Material Selection
The use of tungsten carbide media is a deliberate choice over softer alternatives. The high hardness and density of this alloy are required to generate the specific impact forces needed to fracture and activate the raw ceramic oxides effectively.
Making the Right Choice for Your Goal
To ensure the synthesis of high-quality SSBSN ceramics, align your processing parameters with your desired material outcomes:
- If your primary focus is Phase Purity: Ensure the milling duration is sufficient (24 hours) to achieve the atomic-scale mixing required for a single-phase orthorhombic structure.
- If your primary focus is Reaction Efficiency: Prioritize the use of high-density media like tungsten carbide to maximize specific surface area and lower the activation energy for subsequent heat treatments.
Successful synthesis relies on viewing milling not as a physical step, but as a method of thermodynamic preparation.
Summary Table:
| Key Feature | Role in SSBSN Synthesis |
|---|---|
| Milling Media | Hard Tungsten Carbide balls for high-energy impact and shear forces |
| Duration (24h) | Ensures mechanical activation and accumulation of lattice strain |
| Primary Goal | Atomic-scale mixing and uniform dispersion of raw oxides/carbonates |
| Energy Impact | Lowers activation energy by increasing specific surface area |
| Final Outcome | Formation of a pure, single-phase orthorhombic ferroelectric structure |
Elevate Your Material Synthesis with KINTEK Precision
High-performance ferroelectric ceramics like SSBSN demand more than just standard mixing; they require the extreme mechanical activation that only professional-grade equipment can provide. KINTEK offers state-of-the-art Planetary Ball Mills, Rotary, Vacuum, and CVD systems, all backed by expert R&D and manufacturing to ensure your powders achieve perfect atomic-scale homogeneity.
Whether you need custom-engineered Tungsten Carbide media or high-temperature furnaces for precise sintering, KINTEK is your partner in achieving pure-phase structures. Optimize your laboratory research today—contact us for a tailored solution!
Visual Guide
References
- Anurag Pritam, Susanta Sinha Roy. Multiple relaxation mechanisms in SrBi2Nb2O9 ceramic tweaked by tin and samarium incorporation in assistance with single-step microwave sintering. DOI: 10.1007/s00339-024-07482-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How does the electric arc furnace contribute to carbon neutrality? Decarbonizing Steel with EAF Technology
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure
- Why use nitrogen and flow meters in sludge pyrolysis? Ensuring Superior Biochar Quality and Anaerobic Integrity
- What are the technical advantages of using the molten salt method? Elevate Your Biomass Carbon Support Synthesis
- Why is a constant temperature drying oven used at 120°C for 16 hours for NiCuCe catalysts? Optimize Site Dispersion
- Why is carbon dioxide utilized for the in-situ gasification regeneration of NiCuCe catalysts? Enhance Catalyst Longevity
- Why does the use of a forced-air drying oven often lead to increased particle size? Avoid Silica Agglomeration
- What is the function of an industrial resistance furnace in HPDC magnesium melting? Master Thermal Precision



















