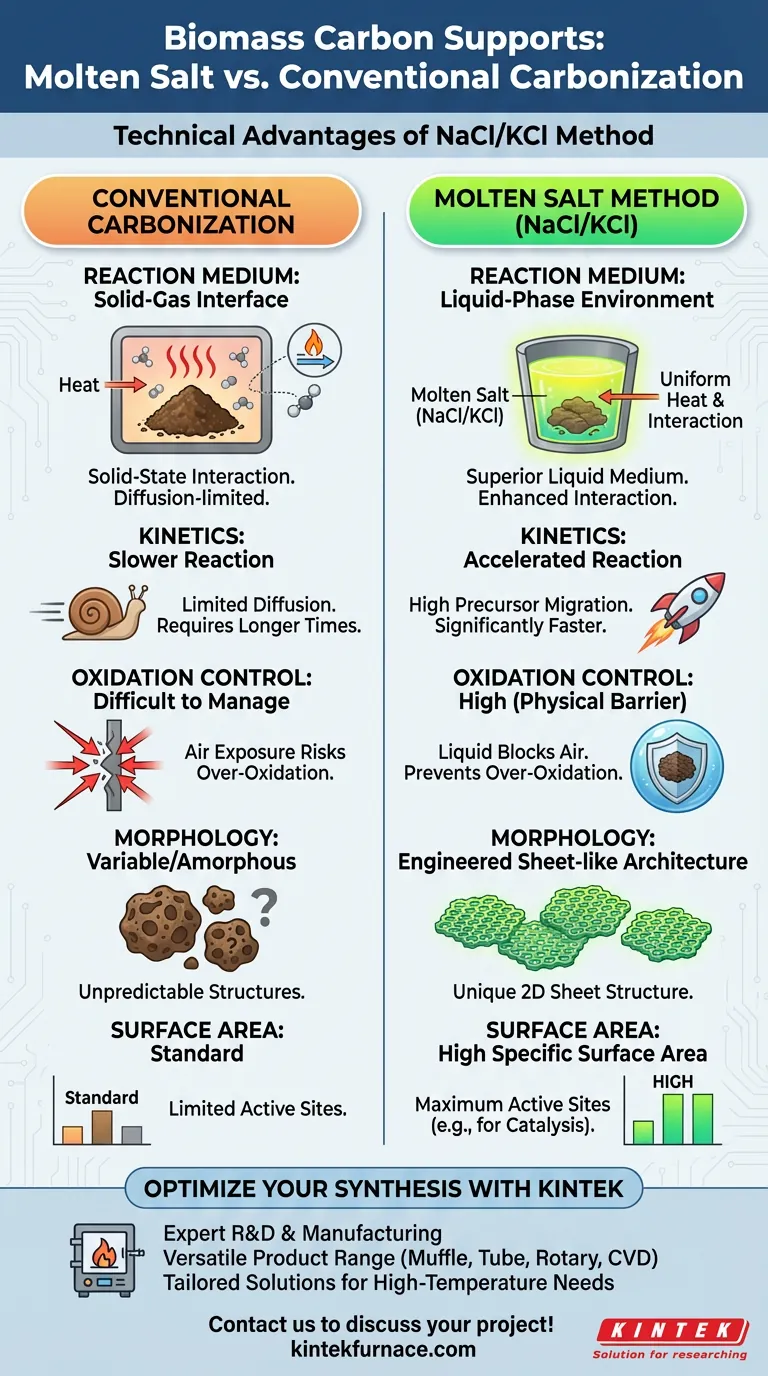
The molten salt method (NaCl/KCl) distinguishes itself from conventional carbonization by creating a liquid reaction medium that fundamentally alters the synthesis process. This technique offers specific technical advantages in reaction speed, environmental control, and the resulting structural morphology of the biomass carbon.
The core advantage of this method lies in the transition from a solid-state reaction to a liquid-phase environment. This medium not only accelerates the carbonization process but also acts as a physical barrier against oxidation, yielding high-performance materials with unique sheet-like architectures.

Mechanisms of Enhanced Synthesis
The Superior Reaction Medium
Conventional carbonization typically relies on solid-gas interactions. In contrast, the NaCl/KCl mixture transforms into a liquid phase at high temperatures.
This liquid environment acts as a superior medium for chemical interactions compared to standard dry heating. It allows for more uniform thermal distribution and material interaction.
Accelerated Reaction Kinetics
The liquid phase actively promotes the dissolution and migration of metal precursors. specifically tungsten sources such as tungsten trioxide derived from ammonium paratungstate.
By facilitating this mobility, the molten salt method significantly accelerates the carbonization reaction. This efficiency is difficult to replicate in solid-state conventional methods where diffusion is limited.
Prevention of Over-Oxidation
A critical challenge in biomass carbonization is controlling the oxidation level. The molten salt liquid environment provides a physical shield that effectively blocks air.
This isolation prevents the over-oxidation of the biomass carbon. Consequently, the method preserves the chemical integrity of the carbon support better than conventional methods exposed to variable gas atmospheres.
Structural and Morphological Benefits
Engineered Surface Architecture
The constraints and interactions imposed by the molten salt medium dictate the final shape of the carbon. This facilitates the formation of a unique sheet-like structure.
High Specific Surface Area
Because of the unique sheet-like morphology and controlled carbonization, the resulting material exhibits a high specific surface area. This characteristic is essential for applications requiring maximum active sites, such as catalysis.
Understanding the Operational Shift
Liquid vs. Solid Phase Processing
Implementing this method requires shifting from simple thermal treatment to managing a liquid salt system.
While conventional methods are chemically simpler, they lack the "active" participation of the medium. The molten salt is not just a heat transfer fluid; it is an active participant that shapes the physical and chemical outcome of the carbon support.
Making the Right Choice for Your Goal
The decision to use the molten salt method should be driven by the specific physical properties you require from your carbon support.
- If your primary focus is Structural Efficiency: Choose the molten salt method to achieve a unique sheet-like structure with a high specific surface area.
- If your primary focus is Material Purity: Rely on this method to block air and prevent the degradation caused by over-oxidation.
- If your primary focus is Reaction Kinetics: Utilize the NaCl/KCl mixture to promote precursor migration and accelerate carbonization.
By leveraging the liquid phase of the NaCl/KCl mixture, you gain precise control over both the reaction speed and the final architecture of your biomass carbon.
Summary Table:
| Feature | Conventional Carbonization | Molten Salt Method (NaCl/KCl) |
|---|---|---|
| Reaction Medium | Solid-Gas Interface | Liquid-Phase Environment |
| Kinetics | Slower (Diffusion-limited) | Accelerated (High precursor migration) |
| Oxidation Control | Difficult to manage | High (Liquid acts as a physical barrier) |
| Morphology | Variable/Amorphous | Engineered Sheet-like Architecture |
| Surface Area | Standard | High Specific Surface Area |
Optimize Your Advanced Material Synthesis with KINTEK
Transitioning from conventional carbonization to advanced molten salt techniques requires precise thermal control and specialized equipment. KINTEK provides the high-performance laboratory solutions needed to master these complex reactions.
Our Value to You:
- Expert R&D & Manufacturing: Our systems are engineered for the rigorous demands of liquid salt and vacuum processing.
- Versatile Product Range: From Muffle and Tube furnaces to specialized Rotary and CVD systems, we cover all your high-temperature needs.
- Tailored Solutions: Every lab is unique; we offer fully customizable furnaces to match your specific biomass carbonization or material synthesis goals.
Whether you are aiming for superior sheet-like structures or accelerated reaction kinetics, KINTEK has the technology to get you there. Contact us today to discuss your unique project requirements!
Visual Guide
References
- Zunming Lu, Xiaofeng Wei. N-S Co-Doped WC Nanoparticles Show High Catalytic Activity in Hydrogen Evolution Reaction. DOI: 10.3390/coatings15060630
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the purpose of using high-purity nitrogen for nano-zinc oxide experiments? Ensure Data Purity & Accuracy
- Why is a forced convection oven necessary in the powder preparation workflow? Optimize Your Thermoelectric Materials
- How do you maintain a vacuum pump? Ensure peak performance and longevity for your lab
- What are the technical advantages of using vacuum-assisted impregnation for 3D LIG/polymer composites? Boost Strength
- Why are high-precision constant temperature baths necessary? Unlock Accurate Fiber Optic Sensor Calibration
- Why must raw kaolin undergo heat treatment for DLP 3D printing? Control Viscosity for Precision Printing
- Why is rapid water quenching necessary for Ce2(Fe, Co)17 alloys? Unlock Peak Magnetocaloric Performance
- What are the functions of an industrial drying furnace vertically installed below a shredder? Efficient LIB Recycling



















