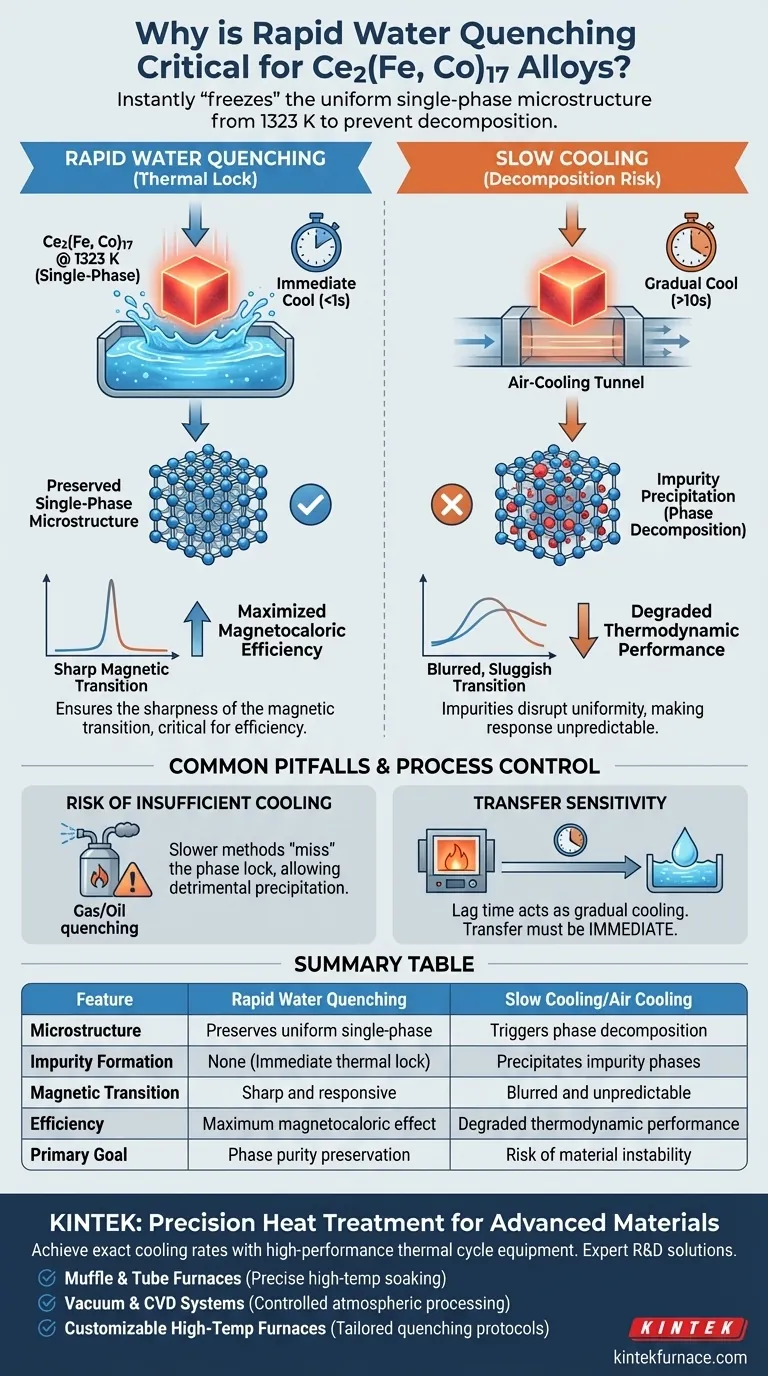
Rapid water quenching is strictly necessary to instantly "freeze" the uniform single-phase microstructure created during heat treatment at 1323 K. This high-speed cooling process bypasses the gradual temperature drop that allows the material to decompose, ensuring that no impurity phases precipitate out of the alloy before it reaches room temperature.
The essential function of rapid water quenching is to preserve the atomic structure established at high temperatures. By preventing phase decomposition, you ensure the sharpness of the magnetic transition, which is the defining factor in maximizing the efficiency of the magnetocaloric effect.

The Mechanics of Microstructural Preservation
Freezing the Single-Phase State
At the heat treatment temperature of 1323 K, Ce2(Fe, Co)17 alloys achieve a uniform, single-phase microstructure. This specific atomic arrangement is optimal for the material's performance.
To retain this structure at room temperature, the cooling process must be instantaneous. Rapid water quenching acts as a thermal lock, solidifying this state before the atoms have time to rearrange.
Preventing Phase Decomposition
If the alloy is allowed to cool gradually, the material enters a zone of instability. During slow cooling, the high-temperature phase naturally begins to decompose.
This decomposition leads to the precipitation of impurity phases. These impurities disrupt the uniformity of the alloy, degrading its final properties.
The Necessity of High Cooling Rates
The physics of this specific alloy demands a cooling rate that only a medium like water can typically provide.
While other methods exist for different metals—such as inert gas or oil quenching used for steels—they may not offer the extreme thermal transfer speed required here. Water quenching ensures the transition happens faster than the diffusion speed of the atoms.
Impact on Magnetic Performance
Sharpening the Magnetic Transition
The purity of the microstructure is directly linked to how the material responds to magnetic fields.
A uniform single-phase structure results in a very sharp magnetic transition. Impurities caused by slow cooling would blur this transition, making the material's magnetic response sluggish or unpredictable.
Maximizing Magnetocaloric Efficiency
The ultimate goal of using Ce2(Fe, Co)17 is often to leverage its magnetocaloric effect (the ability to change temperature under a magnetic field).
This efficiency relies heavily on the sharpness of the magnetic transition. Therefore, rapid quenching is not just a mechanical step; it is the critical enabler of the material's thermodynamic performance.
Common Pitfalls and Trade-offs
The Risk of Insufficient Cooling
The primary trade-off in heat treatment is often between cooling speed and mechanical stress. However, for this specific alloy, compromising on speed is not an option.
Using slower quenching methods (like the gas or oil systems often used for standard steels) creates a risk of "missing" the phase lock. Even a slight delay in cooling can allow enough time for detrimental precipitation to occur.
Process Control Sensitivity
Water quenching is a harsh process that requires precise control.
Because the window to prevent decomposition is so small, the transfer from the furnace to the water bath must be immediate. Any lag time acts effectively as "gradual cooling," undermining the entire heat treatment cycle.
Optimizing for Material Performance
To achieve the best results with Ce2(Fe, Co)17, you must align your quenching strategy with your specific performance goals.
- If your primary focus is Phase Purity: Ensure the transfer from 1323 K to the water quench is immediate to prevent any precipitate formation.
- If your primary focus is Magnetocaloric Efficiency: Prioritize the cooling rate above all else, as the sharpness of the magnetic transition dictates your final efficiency metrics.
The success of your alloy depends entirely on your ability to beat the clock during the cooling phase; speed is the guardian of performance.
Summary Table:
| Feature | Rapid Water Quenching | Slow Cooling/Air Cooling |
|---|---|---|
| Microstructure | Preserves uniform single-phase | Triggers phase decomposition |
| Impurity Formation | None (Immediate thermal lock) | Precipitates impurity phases |
| Magnetic Transition | Sharp and responsive | Blurred and unpredictable |
| Efficiency | Maximum magnetocaloric effect | Degraded thermodynamic performance |
| Primary Goal | Phase purity preservation | Risk of material instability |
Precision Heat Treatment for Advanced Materials
To achieve the exact cooling rates required for Ce2(Fe, Co)17 alloys, your laboratory needs equipment designed for high-performance thermal cycles. KINTEK provides industry-leading solutions backed by expert R&D and manufacturing, ensuring your material research is never compromised by inconsistent cooling.
Our range includes:
- Muffle & Tube Furnaces for precise high-temp soaking.
- Vacuum & CVD Systems for controlled atmospheric processing.
- Customizable High-Temp Furnaces tailored to your quenching protocols.
Whether you are focusing on phase purity or magnetocaloric efficiency, KINTEK offers the reliability you need. Contact us today to optimize your heat treatment process.
Visual Guide
References
- H. Jaballah, Lotfi Bessais. Structural, Magnetic, and Magnetocaloric Properties of Ce2(Fe, Co)17 Compounds: Tuning Magnetic Transitions and Enhancing Refrigeration Efficiency. DOI: 10.3390/ma18091958
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- How does a high-temperature annealing furnace regulate cold-rolled steel? Optimize Manganese Steel Performance
- What is shrinkage in the context of high-temperature materials? Master Dimensional Control for Stronger Parts
- What is the importance of transferring freshly deposited CuO films directly into a 125°C oven? Ensure Film Adhesion
- Why are Cu2O and Ga2O3 targets preferred for CuGaO2 films? Achieving Precision in Delafossite Sputtering
- Why is pressure molding and high-temperature sintering required for UO2-ZrO2? Mastering Material Density
- What is the significance of temperature control precision in high-temperature furnaces for carbon-doped titanium dioxide?
- What is the use of furnace in laboratory? Unlock Precise High-Temperature Control for Material Transformations
- How is an industrial high-temperature furnace utilized for beta-quench treatment of Zr-2.5%Nb alloys?



















