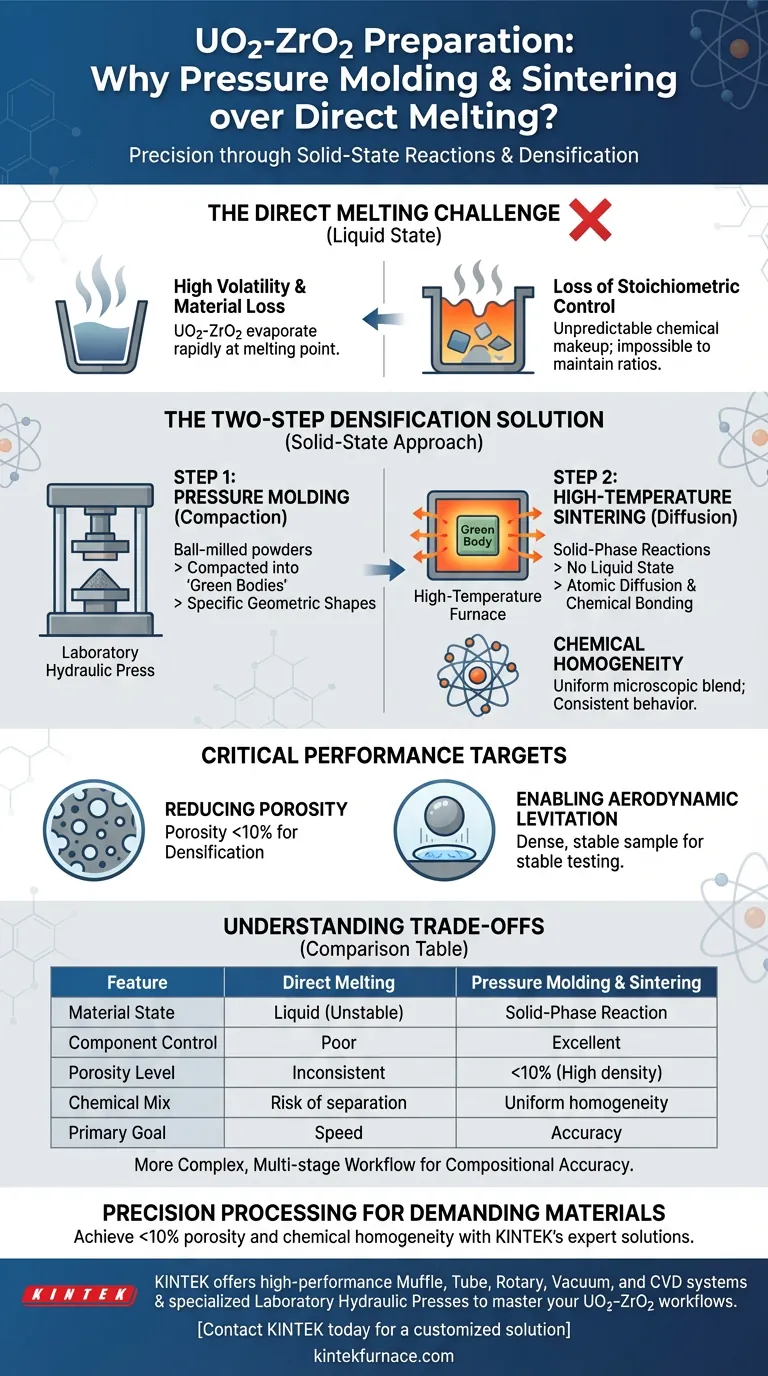
The combination of pressure molding and high-temperature sintering is strictly required for Uranium Oxide and Zirconium Oxide (UO2-ZrO2) mixtures to bypass the severe limitations of direct melting. Because these materials possess extremely high melting points and significant volatility, this solid-state approach is the only reliable method to achieve precise chemical ratios and structural density.
The extreme volatility of UO2-ZrO2 at melting temperatures makes direct liquid processing unreliable for maintaining component ratios. Pressure molding followed by sintering facilitates a stable solid-phase reaction, ensuring chemical homogeneity and high density without the material loss associated with melting.
The Limitations of Direct Melting
The Volatility Challenge
Direct melting is often the standard for mixing materials, but it fails with UO2-ZrO2. These components are highly volatile, meaning they evaporate or degrade rapidly when transitioned to a liquid state.
Loss of Stoichiometric Control
Because of this volatility, it is nearly impossible to control the component ratios during a direct melt. As the material liquefies, the evaporation rates differ, altering the chemical makeup of the final product unpredictable.
The Two-Step Densification Solution
Step 1: Pressure Molding
The process begins with ball-milled powders that are chemically mixed but physically loose. By utilizing a laboratory hydraulic press, these powders are compacted into specific geometric shapes known as "green bodies."
Step 2: High-Temperature Sintering
These green bodies are then processed in a high-temperature furnace. Instead of melting the material to a liquid, the heat induces solid-phase reactions. This allows the atoms to diffuse and bond chemically without reaching the unstable liquid state.
Achieving Chemical Homogeneity
Through these solid-phase reactions, the mixture achieves chemical homogeneity. The components blend uniformly at a microscopic level, ensuring the final material behaves consistently.
Critical Performance Targets
Reducing Porosity
A primary goal of this method is densification. The combination of pressure and sintering reduces the material's porosity to below 10%.
Enabling Aerodynamic Levitation
This low porosity is not just for structural integrity; it is a prerequisite for specific testing environments. A dense, stable sample is necessary to ensure stable performance during aerodynamic levitation testing.
Understanding the Trade-offs
Process Complexity vs. Composition Control
While direct melting is generally a faster, single-step process, it sacrifices control for UO2-ZrO2. The trade-off here is accepting a more complex, multi-stage workflow (milling, pressing, sintering) to guarantee compositional accuracy.
Solid-State Restrictions
This method relies on solid-phase reactions, which are slower than liquid mixing. You must precisely control the sintering furnace parameters to ensure the reaction is complete, as an incomplete reaction will lead to weak points or chemical separation in the sample.
Making the Right Choice for Your Goal
To ensure your UO2-ZrO2 preparation meets experimental requirements, align your process with your specific targets:
- If your primary focus is Chemical Composition: Prioritize the sintering phase to drive solid-phase reactions, as this prevents the volatility loss inherent in melting.
- If your primary focus is Aerodynamic Stability: Focus on the pressure molding parameters to maximize initial density, ensuring final porosity stays below the 10% threshold.
By treating the powder mechanically before heating it chemically, you create a stable, uniform material capable of withstanding extreme testing environments.
Summary Table:
| Feature | Direct Melting Method | Pressure Molding & Sintering |
|---|---|---|
| Material State | Liquid (Unstable) | Solid-Phase Reaction |
| Component Control | Poor due to high volatility | Excellent stoichiometric control |
| Porosity Level | Inconsistent | <10% (High density) |
| Chemical Mix | Risk of separation | Uniform homogeneity |
| Primary Goal | Speed | Compositional accuracy |
Precision Processing for Your Most Demanding Materials
Achieving the <10% porosity required for aerodynamic levitation requires more than just heat—it requires precision equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside specialized laboratory hydraulic presses to master your UO2-ZrO2 workflows. Our customizable lab high-temp furnaces ensure the stable solid-phase reactions necessary for chemical homogeneity.
Ready to elevate your material research? Contact KINTEK today for a customized solution!
Visual Guide
References
- Yaopeng Gong, Weimin Ma. Non-Contact Thermophysical Property Measurements of High-Temperature Corium Through Aerodynamic Levitation. DOI: 10.3390/en18010136
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What is the purpose of using a high-vacuum pump system for NiTi thin films? Ensure Pure Stoichiometry & Performance
- How does an infrared rapid thermal annealing belt furnace affect battery performance? Maximize Efficiency Today
- Why is temperature control precision critical for gas diffusion electrodes? Achieve Perfect PTFE Redistribution
- Why is argon gas preferred over other inert gases? Discover Its Optimal Balance for Industrial Use
- What PPE is suggested for adjusting controls or handling equipment during furnace operation? Essential Gear for Operator Safety
- How does the addition of RhCl3 facilitate the synthesis of RhSeCl crystals? Unlock High-Quality Crystal Growth
- What role do high-temp furnaces play in co-firing SOFCs? Master Ceramic Densification and Sintering
- Why is an optical pyrometer necessary for monitoring nickel-aluminum alloy synthesis? Capture Rapid Thermal Explosions



















