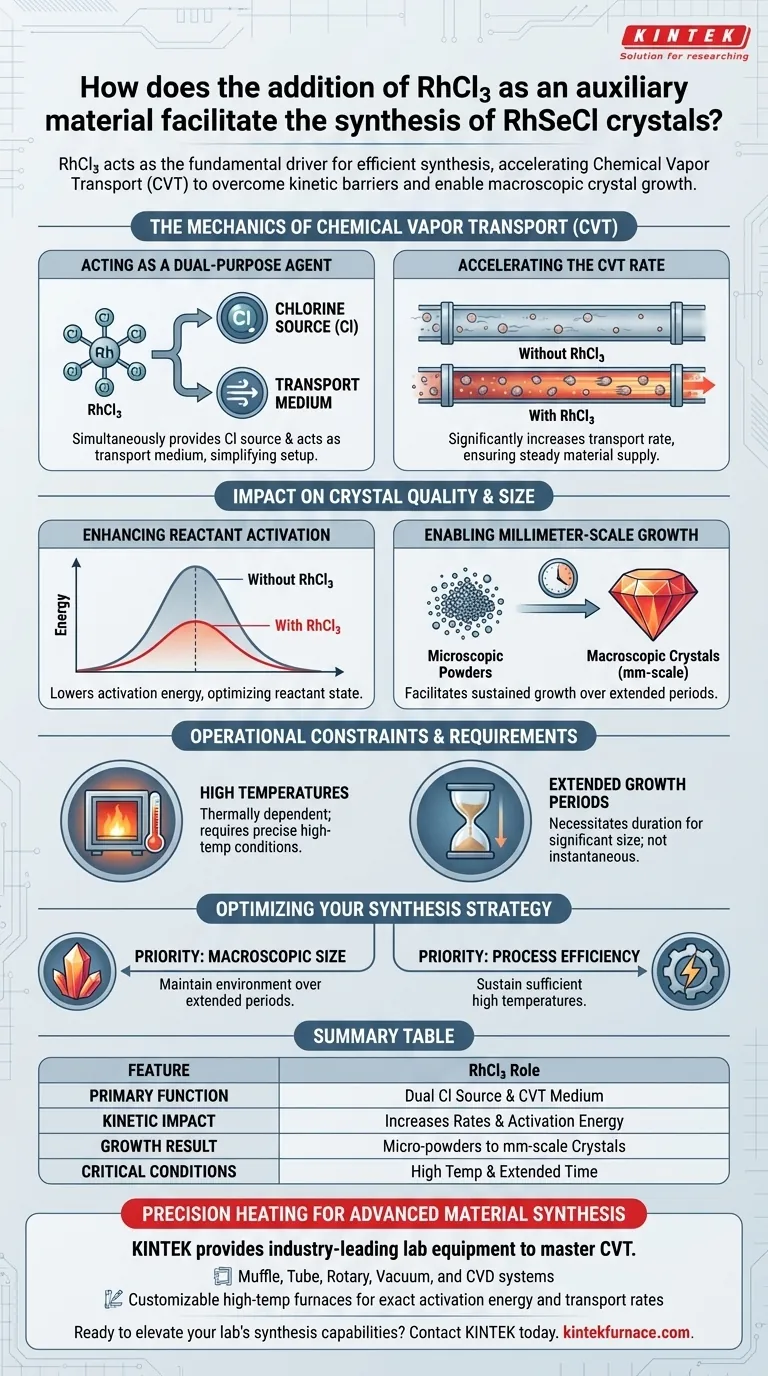
The addition of Rhodium(III) chloride (RhCl3) acts as the fundamental driver for the efficient synthesis of RhSeCl crystals. By functioning simultaneously as a chlorine source and a transport medium, it accelerates the Chemical Vapor Transport (CVT) process to overcome kinetic barriers. This auxiliary material is the key factor enabling the transition from microscopic powders to high-quality, macroscopic crystals.
RhCl3 serves as a vital transport agent that optimizes the reaction environment. By enhancing activation energy and reaction rates under high temperatures, it allows for the sustained growth of larger, millimeter-scale RhSeCl crystals that would otherwise be difficult to achieve.

The Mechanics of Chemical Vapor Transport
Acting as a Dual-Purpose Agent
In the context of RhSeCl synthesis, RhCl3 is not a passive additive. It serves two distinct, critical functions: it provides the necessary chlorine source required for the chemical composition, and it acts as the transport medium.
This dual role simplifies the synthesis setup. It ensures that the necessary chemical components are present while simultaneously driving the physical movement of mass required for crystal formation.
Accelerating the CVT Rate
The presence of RhCl3 has a direct impact on the kinetics of the system. It significantly increases the rate of Chemical Vapor Transport (CVT).
By speeding up the transport of vaporized species, RhCl3 ensures a steady supply of material to the crystallization zone. This continuous feed is essential for sustaining crystal growth without interruption.
Impact on Crystal Quality and Size
Enhancing Reactant Activation
For crystallization to occur, reactants must overcome a specific energy barrier. RhCl3 plays a pivotal role here by enhancing the activation energy of the reactants.
This modification of the energy landscape makes the chemical transformation more efficient. It ensures that the reactants are in an optimal state to bond and form the desired crystal lattice structure.
Enabling Millimeter-Scale Growth
The ultimate tangible benefit of using RhCl3 is visible in the physical dimensions of the product. Without an effective transport agent, synthesis often results in fine powders or micro-crystals.
RhCl3 facilitates the growth of larger, millimeter-scale crystals. It stabilizes the process enough to support crystal formation over extended growth periods, allowing the lattice to expand well beyond microscopic limits.
Operational Constraints and Requirements
Reliance on High Temperatures
The facilitating effects of RhCl3 are not automatic; they are thermally dependent. The enhancement of activation energy and transport rates is triggered specifically under high-temperature reaction conditions.
You cannot achieve these results at ambient temperatures. Precise thermal control is required to activate the RhCl3 and initiate the transport mechanism.
The Necessity of Time
While RhCl3 increases the rate of transport, achieving significant size still requires duration. The synthesis relies on extended growth periods.
RhCl3 makes large crystals possible, but it does not make them instant. The process sacrifices rapid throughput for the sake of achieving superior physical dimensions and structural integrity.
Optimizing Your Synthesis Strategy
To maximize the utility of RhCl3 in your crystal growth experiments, consider the following technical priorities:
- If your primary focus is achieving macroscopic size: Maintain the reaction environment over extended periods to allow RhCl3 to drive the continuous accumulation of material into millimeter-scale formations.
- If your primary focus is process efficiency: Ensure your setup achieves and sustains sufficient high temperatures to fully leverage the ability of RhCl3 to enhance activation energy and transport rates.
By effectively utilizing RhCl3 as a transport medium, you transform a standard synthesis process into a robust method for creating high-quality, macroscopic RhSeCl crystals.
Summary Table:
| Feature | Role of RhCl3 in RhSeCl Synthesis |
|---|---|
| Primary Function | Acts as both a chlorine source and a Chemical Vapor Transport (CVT) medium. |
| Kinetic Impact | Increases reaction rates and enhances reactant activation energy. |
| Growth Result | Facilitates the transition from micro-powders to millimeter-scale macroscopic crystals. |
| Critical Conditions | Requires sustained high-temperature environments and extended growth periods. |
Precision Heating for Advanced Material Synthesis
High-quality RhSeCl crystal growth depends on precise thermal control and stable high-temperature environments. KINTEK provides the industry-leading lab equipment necessary to master the Chemical Vapor Transport process.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our high-temp furnaces are fully customizable to meet the unique needs of your research, ensuring you achieve the exact activation energy and transport rates required for superior material production.
Ready to elevate your lab's synthesis capabilities? Contact KINTEK today to find the perfect furnace for your application.
Visual Guide
References
- Kefeng Liu, Huiyang Gou. Optimized Synthesis and Characterization of Janus RhSeCl with Uniform Anionic Valences, Nonlinear Optical and Optoelectronic Properties. DOI: 10.1002/advs.202505279
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Vacuum Induction Melting Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- Why is precision constant temperature control required during the hardening stage of geopolymer mortar? Guide to Success
- What is the significance of 1200 °C in ZrO2:Ti synthesis? Unlock Phase Purity in High-Performance Ceramics
- Why is a high vacuum necessary for solar absorbers? Ensure Precise Optical Properties in Thin Film Coating
- Why are acid washing and vacuum drying ovens required after carbon activation? Unlock Maximum Purity and Pore Access
- How do thermal stripping tools and heating equipment facilitate solar panel recycling? High-Value Glass Recovery Guide
- Why is a high-purity argon flow control system essential? Ensure Precision in Metallurgy Simulations
- What is the function of a high-temperature heat treatment furnace? Optimize AlCuCrFe2NiTi0.25 Alloy Properties
- What is the purpose of high-purity argon in Fe60Co10-xNi15Cr15Six alloy preparation? Ensure Purity for Laser Cladding



















