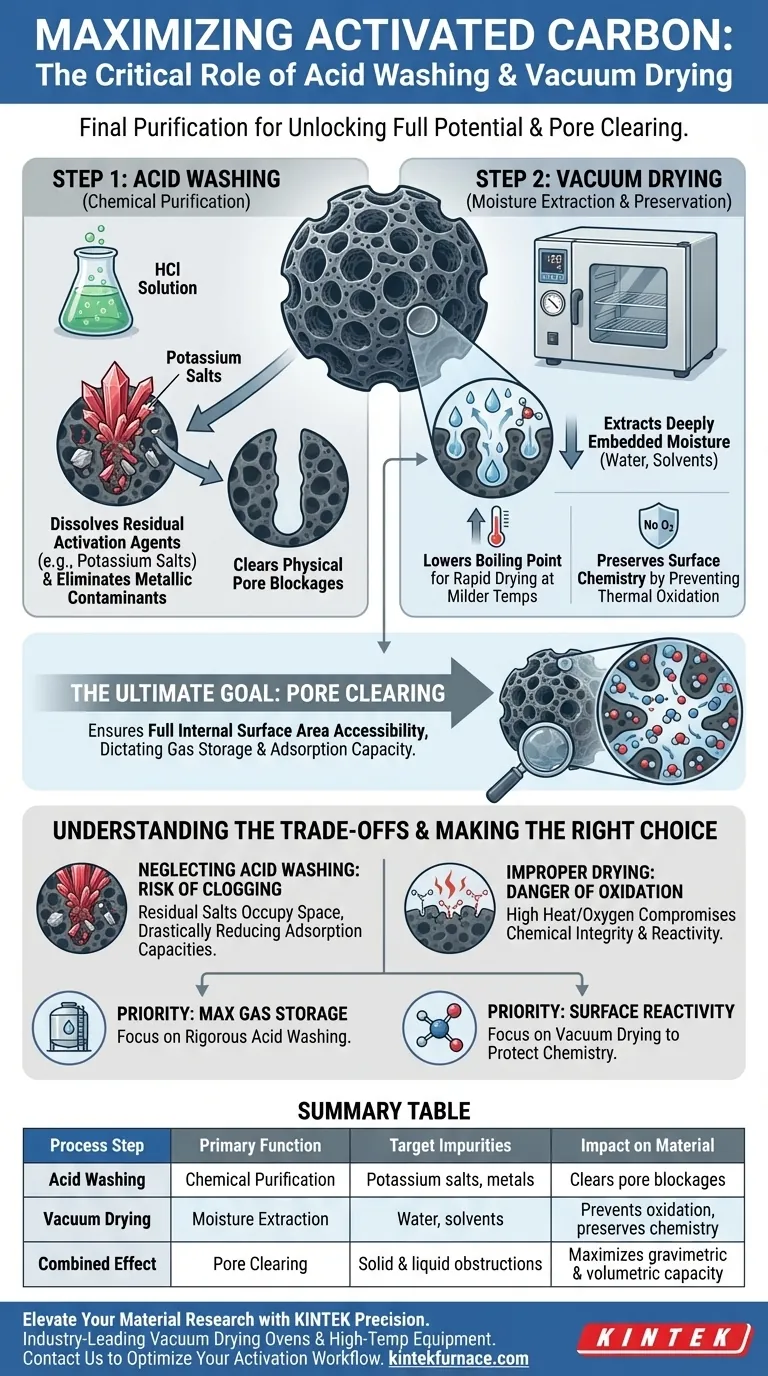
Acid washing and vacuum drying serve as the final purification stage required to unlock the full potential of activated carbon materials. Acid washing chemically dissolves residual activation agents—specifically potassium salts and metallic impurities—while vacuum drying efficiently removes moisture and solvents from deep within the pore structure.
The ultimate goal of this two-step sequence is pore clearing. By removing physical blockages—whether solid salts or liquid moisture—you ensure the internal surface area is fully accessible, which directly dictates the material's capacity for gas storage and adsorption.
The Role of Acid Washing
Dissolving Solid Impurities
The activation process often utilizes chemical agents, leaving behind residues such as potassium salts. Acid washing, typically using a hydrochloric acid (HCl) solution, is the primary method for dissolving these salts.
Eliminating Metallic Contaminants
Beyond activation salts, the carbon precursor or processing equipment may introduce metallic impurities. Acid washing acts as a chemical scour, leaching these metals out of the carbon matrix to ensure a high-purity end product.
The Necessity of Vacuum Drying
Extracting Deeply Embedded Moisture
After washing, the porous structure is saturated with water or solvents like ethanol. A vacuum drying oven, operating at temperatures around 120 °C, is used to forcefully evaporate this trapped liquid from the micro- and mesopores.
Lowering the Boiling Point
Applying a vacuum reduces the pressure surrounding the material, which lowers the boiling point of water and solvents. This allows for rapid drying without requiring excessive heat that could damage the carbon structure.
Preserving Surface Chemistry
Standard high-heat drying can lead to thermal oxidation, which destroys beneficial active functional groups on the carbon's surface. Vacuum drying mitigates this risk by removing oxygen from the environment and allowing for effective drying at milder temperatures.
Understanding the Trade-offs
The Risk of Incomplete Purification
If you neglect acid washing, residual salts will physically occupy the pore space. This "clogging" drastically reduces the gravimetric and volumetric adsorption capacities, rendering the material less effective for gas storage applications.
The Danger of Improper Drying
Skipping the vacuum step or drying in an oxygen-rich environment can compromise the material's chemical integrity. While the pores might eventually dry, the surface chemistry may be altered by oxidation, changing how the carbon interacts with target gases or adsorbates.
Making the Right Choice for Your Goal
To maximize the utility of your carbon materials, tailor your post-processing to your specific performance metrics:
- If your primary focus is maximum gas storage capacity: Prioritize rigorous acid washing to ensure every pore is completely cleared of salts and metallic obstructions.
- If your primary focus is surface chemical reactivity: Ensure you use a vacuum drying oven to protect sensitive functional groups from thermal oxidation during the drying phase.
By meticulously clearing the internal architecture of the carbon, you transform a raw processed material into a high-performance adsorbent.
Summary Table:
| Process Step | Primary Function | Target Impurities | Impact on Material |
|---|---|---|---|
| Acid Washing | Chemical Purification | Potassium salts, metallic contaminants | Clears physical pore blockages |
| Vacuum Drying | Moisture Extraction | Water, solvents (ethanol) | Prevents oxidation & preserves chemistry |
| Combined Effect | Pore Clearing | Solid & liquid obstructions | Maximizes gravimetric & volumetric capacity |
Elevate Your Material Research with KINTEK Precision
Don't let residual impurities compromise your activated carbon's performance. KINTEK provides industry-leading Vacuum Drying Ovens and specialized high-temperature lab equipment designed to preserve sensitive surface chemistries while ensuring deep-pore purification.
Backed by expert R&D and world-class manufacturing, we offer fully customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to the unique needs of carbon material scientists. Whether you are scaling up gas storage research or refining high-purity adsorbents, our technical team is ready to assist.
Contact KINTEK Today to Optimize Your Activation Workflow
Visual Guide
References
- Nawaf Albeladi, Robert Mokaya. Ultra-high surface area ionic-liquid-derived carbons that meet both gravimetric and volumetric methane storage targets. DOI: 10.1039/d3ee03957a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What role does a pyrolysis furnace play in preparing graphene nanosheets? Master High-Value Plastic Transformation
- What processing conditions does an industrial heating furnace provide during hot forging? Optimize Fe-Mn-Si Alloys
- What is the purpose of an industrial oven for powder pre-treatment? Ensure Accurate Silica Analysis
- How is the thermal stability of KBaBi compounds evaluated? Discover Precise XRD & Heat Treatment Limits
- What is the function of wet ball milling in the synthesis of SPAN? Optimize Your Sulfur Content Through Deep Mixing
- How does the use of carbon dioxide and a flow meter impact the physical activation of biochar? Master Pore Development
- What is the purpose of using a rotary evaporator or a vacuum drying oven? Preserving SiC Powder Quality Post-Milling
- Why is a vacuum drying oven required for precursor mixtures? Achieve Stable, High-Quality Powder Processing



















