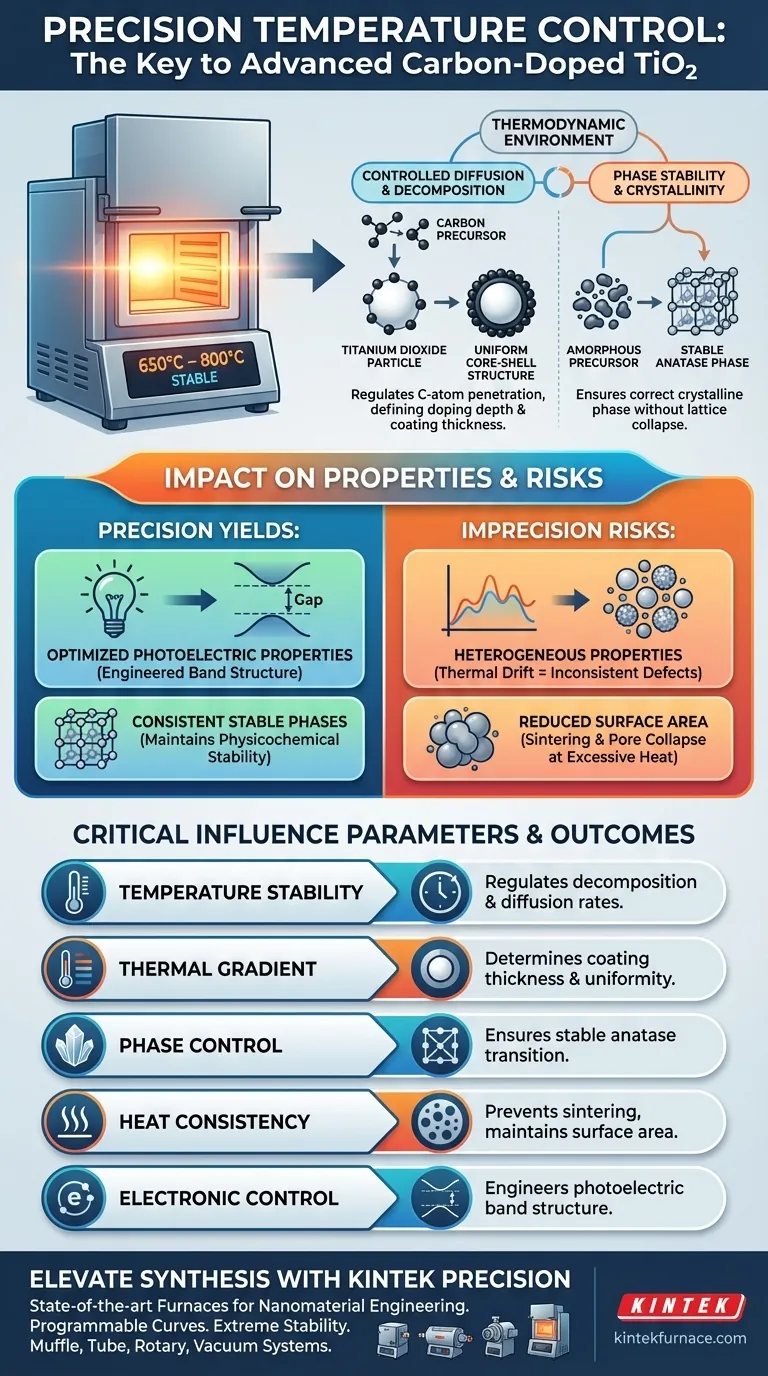
Precise temperature control establishes the specific thermodynamic environment required to successfully synthesize carbon-doped titanium dioxide. By maintaining strict thermal gradients, typically between 650°C and 800°C, the furnace regulates the decomposition of carbon precursors and drives their diffusion onto the titanium dioxide surface, directly dictating the material's final structural and photoelectric quality.
Temperature precision is the governing variable that controls the thickness of carbon coatings and the depth of doping in core-shell structures. Without this thermodynamic stability, it is impossible to reliably engineer the photoelectric properties necessary for advanced applications.

The Thermodynamic Role of Precision
Controlling Carbon Diffusion
In the preparation of carbon-doped titanium dioxide, the furnace does more than simply heat the sample. It creates a thermodynamic environment that forces carbon precursors to decompose at a controlled rate.
This controlled decomposition allows carbon atoms to diffuse effectively onto the titanium dioxide surface. The precision of the heat directly influences how deeply these atoms penetrate, determining the doping depth.
Engineering Core-Shell Structures
The primary goal of this thermal process is often the formation of specific core-shell structures. The furnace must maintain temperatures within the 650°C to 800°C range to facilitate this formation.
Variations in temperature control will alter the coating thickness of the shell. Precise gradients ensure that the carbon shell forms uniformly around the titanium dioxide core, rather than aggregating randomly or failing to adhere.
Impact on Material Properties
Defining Photoelectric Characteristics
The physical structure resulting from the firing process correlates directly to the material's performance. The specific coating thickness and doping depth achieved through precise heating determine the final photoelectric properties of the nanoparticles.
If the temperature deviates, the electronic band structure may not change as intended, rendering the doping ineffective for its target application.
Managing Phase Stability
While the primary focus is on carbon doping, temperature precision also dictates the crystalline phase of the titanium dioxide itself. As seen in similar doping processes (such as with Cerium), precise heating curves are required to transform amorphous precursors into stable phases like anatase.
Controlled heating ensures that the lattice accepts the dopant without collapsing or converting into an undesirable crystalline phase. This balance is critical for maintaining physicochemical stability alongside the new doped characteristics.
Understanding the Trade-offs
The Risk of Thermal Drift
In high-precision material science, even minor fluctuations can ruin a batch. Just as thermal stability is vital in electrochemical testing to prevent property drift, furnace instability during synthesis can lead to heterogeneous material properties.
If the temperature fluctuates during the diffusion window, the concentration of carbon carriers may vary across the sample. This results in inconsistent structural defects and unpredictable catalytic behavior.
High Temperature vs. Surface Area
Higher temperatures generally facilitate better diffusion and crystallinity, but there is a point of diminishing returns. Excessive heat can lead to sintering, which reduces the specific surface area and pore volume.
While activation processes (like those used in activated carbon) use high heat to create pores, uncontrolled high heat in doping can collapse the very micro-structures you are trying to engineer.
Making the Right Choice for Your Goal
To achieve the best results in carbon-doped titanium dioxide preparation, align your furnace parameters with your specific material objectives.
- If your primary focus is Photoelectric Efficiency: Prioritize stability in the 650°C–800°C range to strictly control coating thickness and doping depth.
- If your primary focus is Structural Homogeneity: Ensure your furnace offers programmable heating curves to manage phase transitions and prevent the formation of mixed phases.
The precision of your thermal control is the single most important factor in translating a chemical precursor into a functional, high-performance nanomaterial.
Summary Table:
| Parameter | Influence on Material Outcome |
|---|---|
| Temperature Stability | Regulates carbon precursor decomposition and diffusion rates |
| Thermal Gradient | Determines coating thickness and uniformity of core-shell structures |
| Phase Control | Ensures transition to stable anatase phases without lattice collapse |
| Heat Consistency | Prevents sintering and maintains high specific surface area/pore volume |
| Electronic Control | Directly engineers the final photoelectric band structure properties |
Elevate Your Material Synthesis with KINTEK Precision
Don't let thermal drift compromise your research. KINTEK provides state-of-the-art Muffle, Tube, Rotary, and Vacuum furnaces specifically designed to meet the rigorous thermodynamic demands of nanomaterial engineering.
Backed by expert R&D and manufacturing, our systems offer the programmable heating curves and extreme temperature stability required for precise carbon-doping and phase management. Whether you need a standard solution or a fully customizable high-temperature system, KINTEK is your partner in achieving superior structural and photoelectric performance.
Ready to optimize your doping process? Contact our experts today to find the perfect furnace for your lab.
Visual Guide
References
- Dominik Eitel, Uta Helbig. Structural Characterization of Carbon‐Doped and Carbon‐Coated TiO <sub>2</sub> Core–Shell Nanoparticles. DOI: 10.1002/admi.202500770
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are batch catalytic debinding ovens used for? Speed Up MIM/CIM with Low-Temp Debinding
- What is the objective of GC-MS analysis on bio-oil? Unlock Chemical Value and Industrial Utility
- Why is a precision constant temperature drying oven required for the impregnation modification process of activated carbon?
- What is the purpose of adding calcium fluoride (CaF2) in magnesium production? Accelerate Your Vacuum Carbothermal Reduction
- What is the importance of a laboratory oven's programmed heating for epoxy-polyimide curing? Essential Thermal Control
- Why is precision constant temperature control required during the hardening stage of geopolymer mortar? Guide to Success
- What are the advantages of using batch furnaces? Boost Your Process Flexibility and Precision
- What are the advantages of using TGA-MS over standalone TGA for activated carbon? Unlock Deep Chemical Insights



















